|
|
|
|
|
Disseminated Geotrichosis in Prolonged Neutropenia
|
|
|
|
Arun Prabhakaran Nair1, Sreethish Sasi2, Rakesh Parakadavathu1, Mohammed Abukhattab1, Samar Mahmoud Hashim1, Suleiman Abu Jarir1, Muna Al-Maslamani1 1Department of Infectious Diseases, Communicable Disease Center; 2Department of Internal Medicine and Medical Education, Hamad General Hospital, Hamad Medical Corporation, Doha, Qatar. |
|
|
|
|
|
Corresponding Author:
|
|
Dr. Sreethish Sasi Email: sreethishsasi@gmail.com |
|
|
|
|
|
|
|
|
Received:
05-MAR-2020 |
Accepted:
17-MAY-2020 |
Published Online:
10-JUN-2020 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Background: Disseminated fungemia due to non-candida yeast is emerging as an opportunistic infection in hematological malignancies with prolonged neutropenia. Invasive infections by Geotrichum spp. are extremely rare and constitute only about 1% of all non-candida yeasts. The mortality in such patients is high and is seldom reported in the literature. Case Report: Here we describe a middle-aged man with treatment-resistant Hodgkin's lymphoma who developed invasive Geotrichum capitatus infection during his neutropenic phase, with poor response to combination therapy with voriconazole and amphotericin B. Conclusion: Geotrichum capitatus is responsible for fatal fungemia in patients with prolonged neutropenia complicating hematological malignancies. Geotrichum species are considered intrinsically resistant to echinocandins. Fluconazole minimum inhibitory concentration (MIC) for this species remains high. A high index of suspicion is required in this patient-group to identify disseminated geotrichosis as delay in treatment worsens mortality. We recommend the use of voriconazole with amphotericin B in immunocompromised patients with a high risk of disseminated fungemia. |
|
|
|
|
|
Keywords :
|
Amphotericin B, Candida, Fungemia, Geotrichosis, Neutropenia, Voriconazole.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffe4e22d0000006006000001000700 6go6ckt5b5idvals|1999 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Invasive infections by non-candida yeasts, which represent less than 5% of the yeast isolates, are emerging as a cause of opportunistic infection with high mortality in immunocompromised. Cryptococcus neoformans (31.2%), Saccharomyces spp. (11.8%), Trichosporon spp. (10.6%) and Rhodotorula spp. (4.1%) were the most commonly identified non-candida yeast species [ 1]. Invasive infections by Geotrichum spp. are sporadic and constitute only about 1% of all non-candida yeasts. Three different species of geotrichum were described based on morphological characters and clinical spectrum, namely Geotrichum candidum, Geotrichum capitatus and Geotrichum clavatum. Geotrichum capitatus is a non-fermentative, non-encapsulated, urease-negative yeast belonging to Ascomycota division. It is found in various environmental sources, part of the healthy microbiota of human skin, and frequently isolated from sputum and the digestive tract of healthy people [ 2]. Geotrichum capitatus is responsible for fatal fungemia in patients with prolonged neutropenia complicating hematological malig-nancies [ 3]. In immunocompromised hosts, Geotrichum spp. should not be considered as a colonizing organism. In the setting of breakthrough fungemia in a patient on anti-fungal prophylaxis, it is crucial to understand the resistance pattern of opportunistic pathogens when choosing empirical anti-fungals for treatment. Amphotericin B with flucytosine is the recommended antifungal for invasive geotrichosis. Even with appropriate treatment, invasive geotrichum infections are associated with high mortality [ 4].
Case Report
We present the case of a 56-year-old gentleman who was diagnosed with Hodgkin’s lymphoma 25 years ago, complicated with multiple relapses and treatment-related T-cell lymphoma inspite of several cycles of chemo-radiation and splenectomy. He voluntarily opted to stop all active treatments in the last two years. In January 2017, he was admitted to the hospital with neutropenia, hemolytic anemia and sepsis, diagnosed as pseudomonas bacteremia of an unknown source, treated with meropenem then cefepime and later levofloxacin pending recovery from neutropenia. Five days post-discharge from the hospital, he came back with fever, drowsiness, and generalized body rash. He was cachexic, febrile, icteric, and neck was supple. He was alert and hemodynamically stable without any neurological deficit. Examination of chest showed decreased air entry at bases with bilateral crackles. Head, arms, trunk and thighs revealed multiple non-blanching purpuric skin rashes most of which were nodular and others with a plaque-like morphology [Fig.1]. Palms and soles were spared. There were petechiae on the palate. The abdomen was soft and non-tender without organomegaly. Cardiovascular examination was unremarkable. He required supplemental oxygenation through a non-rebreather mask. The patient refused invasive procedures like a lumbar puncture or airway intubation. He was suspected of having sepsis due to hospital-acquired pneumonia or urinary tract infection and was started on piperacillin-tazobactam and vancomycin pending sepsis workup.
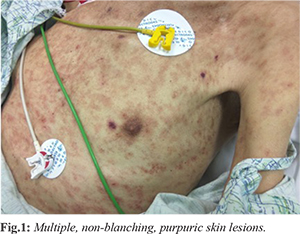
Laboratory evaluation was notable for absolute neutropenia with WBC count of 0.2 cells/mL, hemoglobin of 5.4 gm/dL and platelets of 5000/µL. Renal functions and liver enzymes were within normal limits, but the total bilirubin was high (59.8 µmol/L) with predominantly indirect bilirubin suggesting intravascular hemolysis. Chest X-ray showed right lower-lobe infiltrates with para-pneumonic effusion. A day after, his condition worsened with hypotension requiring inotropes and an increase in oxygen requirements. Peripheral venous sample grew E-coli which was an ESBL producer. Urine showed 21 WBC/µL and 104 CFU/mL of Geotrichum species. He was started on meropenem based on the susceptibility and vancomycin was discontinued. Considering the possibility of disseminated fungal infection in neutropenia and the isolation of Geotrichum from urine, he was given liposomal amphotericin B at a dose of 5 mg/kg daily. Viral PCR for adeno, CMV, Parvo, HHV6, HHV7, measles and enterovirus were negative. Aspergillus galactomannan assay from blood was positive. Skin biopsy was deferred due to thrombocytopenia. Skin scrapings from the nodular lesions showed the presence of Blankophor stain positive fungal elements, but no fungus could be isolated. Repeated blood cultures from the central and peripheral lines grew Geotrichum capitatum [Fig.2], which was identified by MALDI-TOF as Magnusiomyces capitatus, sensitive to amphotericin B, fluconazole, voriconazole and flucytosine and resistant to caspofungin. The central line was removed and intravenous voriconazole was added. Blood cultures repeated after 3 days of antifungal therapy still grew Geotrichum. His course was complicated with Clostridium difficile associated diarrhea which was treated with intravenous metronidazole and oral vancomycin. He recovered from neutropenia after 4 days of admission, but his clinical course continued to deteriorate, and he succumbed to the illness on tenth day of hospitalization.
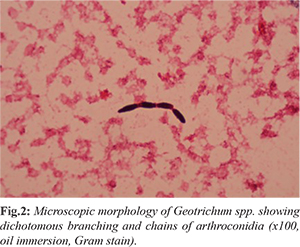
Discussion
G. capitatum is an opportunistic pathogenic fungus which causes infection mainly in patients with hematologic malignancies. Systemic infection by G. capitatum has been reported from Europe, Japan, China and the Mediterranean area [ 5], but this is the first reported case from Qatar. The above case highlights the complexity of recognizing and managing invasive infections due to rare fungi. The clinical manifestations of G. capitatum infection are similar to other fungal infections. Liver and spleen abscesses, pancreatitis, funguria, acute renal failure due to glomerular obstruction by fungus, vertebral osteomyelitis and brain abscess were reported in immunocompromised patients. Skin lesions resembling disseminated candidiasis were reported in patients with hematological malignancies. Most of the invasive infections are diagnosed by blood culture. In Sabouraud’s agar, colonies of Geotrichum species exhibit moderately rapid growth, producing off-white to cream colored colonies with matt appearance and isolates produce true hyphae, pseudohyphae, blastoconidia, arthroconidia and annelloconidia. Colonies grow best at around 25°C to 30°C and are inhibited at 37°C. Species identification can be done with the help of MALDI-TOF-MS [ 6]. Aspergillus galactomannan assay may cross-react with soluble antigens of Geotrichum species as seen in this case [ 7]. Optimal source control is the key to success in the management of Geotrichum infections. One case of G. candidum fungaemia responded well to the removal of a central venous catheter in the absence of antifungal therapy [ 8]. Clinical data regarding the optimal choice of anti-fungal is limited. Due to anti-fungal MIC variability among G. capitatum isolates and among other fungal pathogens with which it may be confused, in vitro MIC determination is essential to guide effective treatment. Based on in-vitro data, amphotericin with or without flucytosine is the most appropriate choice [ 9]. Some authors have recommended voriconazole with amphotericin for combination therapy. Geotrichum species are considered intrinsically resistant to echinocandins and there are case reports of breakthrough infections associated with their use in haematological malignancies [ 10]. In this case, susceptibility test results suggested G. capitatum was sensitive to amphotericin-B, fluconazole, voriconazole and flucytosine and resistant to caspofungin. Some authors have mentioned the use of granulocyte-colony stimulating factors, granulocyte transfusions and interferon-gamma in addition to antifungal treatment with varying success [ 11].
Conclusion
The unavailability of proper guidelines limits empiric therapy for suspected severe invasive fungal infections. Our patient recovered from neutropenia, but fungemia persisted inspite of source control and dual anti-fungal drugs. Considering that all non-Candida yeast except Rhodotorula spp. are sensitive to voriconazole and all except Trichosporon spp. are susceptible to amphotericin B, we recommend the use of voriconazole with amphotericin B in immunocompromised patients with a high risk of disseminated fungemia. Even with appropriate treatment the prognosis remains bad.
Contributors: APN and SS was responsible for the writing the manuscript and literature review; RP, SAJ and MLM were involved in the review of literature and final editing; MA and SMH were part of the expert infectious diseases team who treated the patient, provided us with insights about the course of the patient, involved in manuscript writing and obtaining clinical images. SS will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study. Funding: None; Competing interests: None stated.
References - Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Bijie Dzierzanowska HD, et al. Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2007: 10.5-year analysis of susceptibilities of non-candidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2009;47:117-123.
- Chitasombat MN, Kofteridis DP, Jiang Y, Tarrand J, Lewis RE, Kontoyiannis DP. Rare opportunistic (non-candida, non-cryptococcus) yeast bloodstream infections in patients with cancer. J Infect. 2012;64:68-75.
- Kassamali H, Anaissie E, Ro J, Rolston K, Kantarjian H, Fainstein V, et al. Disseminated Geotrichum candidum infection. J Clin Microbiol. 1987;25:1782-1783.
- Johnson MD, Perfect JR. Use of antifungal combination therapy: Agents, order, and timing. Curr Fungal Infect Rep. 2010;4:87-95.
- Binder U, Lass-Florl C. Epidemiology of invasive fungal infections in the mediterranean area. Mediterr J Hematol Infect Dis. 2011;3.
- Gao GX, Tang HL, Zhang X, Xin XL, Feng J, Chen XQ. Invasive fungal infection caused by Geotrichum capitatum in patients with acute lymphoblastic leukemia: a case study and literature review. Int J Clin Exp Med. 2015;8:14228-14235.
- Giacchino M, Chiapello N, Bezzio S, Franca F, Paola F, Alda A, et al. Aspergillus galactomannan enzyme-linked immunosorbent assay cross-reactivity caused by invasive Geotrichum capitatum. J Clin Microbiol. 2006;44:3432-3434.
- Sheehy TW, Honeycutt BK, Spencer JT. Geotrichum Septicemia. JAMA. 1976;235:1035-1037.
- Cofrancesco E, Viviani MA, Boschetti C, Tortorano AM, Balzani A, et al. Treatment of chronic disseminated Geotrichum capitatum infection with high cumulative dose of colloidal amphotericin B and itraconazole in a leukaemia patient. Mycoses. 1995;38:377-384.
- Etienne A, Datry A, Gaspar N, Morel V, Delabesse E, Lmimouni B, et al. Successful treatment of disseminated Geotrichum capitatum infection with a combination of caspofungin and voriconazole in an immunocompromised patient. Mycoses. 2008;51:270-272.
- Gea-Banacloche J. Granulocyte transfusions: A concise review for practitioners. Cytotherapy. 2017;19:1256-1269.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Prabhakaran Nair A, Sasi S, Parakadavathu R, Abukhattab M, Hashim SM, Abu Jarir S, Al-Maslamani MDisseminated Geotrichosis in Prolonged Neutropenia.JCR 2020;10:116-119 |
|
Prabhakaran Nair A, Sasi S, Parakadavathu R, Abukhattab M, Hashim SM, Abu Jarir S, Al-Maslamani MDisseminated Geotrichosis in Prolonged Neutropenia.JCR [serial online] 2020[cited 2025 Dec 17];10:116-119. Available from: http://www.casereports.in/articles/10/2/Disseminated-Geotrichosis-in-Prolonged-Neutropenia.html |

|
|
|
|
|