6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff2443380000006507000001000600
6go6ckt5b5idvals|3126
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
This report describes the management of a primary aorto-enteric fistula (PAEF), a rare but life-threatening pathology, defined as a communication between the aorta and gastrointestinal tract. First described in 1829, the reported incidence is 0.04-0.07%, with approximately 350 reported cases [
1]. Proximity with the enteric system makes the fistulous communication common with third and the fourth part of duodenum, followed by jejunum and ileum. Clinically, aorto-enteric fistula presents with triad of symptoms, first explained by Sir Astley Cooper, however a ‘herald gastrointestinal bleed’ can be catastrophic [
2]. In view of rarity of the entity, management protocols are based on case reports and case series. In this case we revascularized the lower limbs with an extra-anatomical bypass, in view of a soiled retroperitoneum, and resected the mycotic aortic aneurysm [
3].
Case Report
A 62-year-old gentleman presented with complaints of diffuse pain abdomen, low grade fever and vomiting of one week duration. He had one episode of bleeding per rectum one day prior to his hospital visit. He also gave history of and unquantifiable weight loss and anorexia of one week duration. He was being evaluated for his fever and pain abdomen when the episode of bleeding per rectum occurred and in anticipation of it being a ‘herald bleed’ his treating clinician ordered a computed tomography angiography (CTA) which revealed an infrarenal saccular abdominal aortic aneurysm with loss of fat planes, with abutting fourth part of duodenum. He was hence referred to this vascular surgery centre for emergency management. On examination he had diffuse abdominal pain, with no guarding or rigidity. He had tachycardia and hypotension. On palpation of abdomen a pulsatile peri-umbilical swelling (8×6 cm) was palpable. All peripheral pulses were palpable.
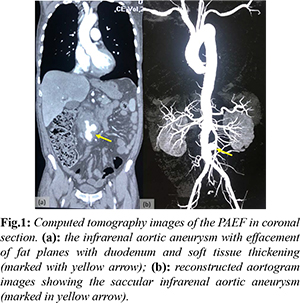
His haematological reports revealed leucocytosis (total leucocyte count: 18.12×103/L) and anemia (hemoglobin: 7 mmol/L). Other markers of inflammation like erythrocyte sedimentation rate and serum procalcitonin were also raised. A CTA revealed circumscribed thickening of abdominal aorta (93 mm long segment) with fusiform dilatation (52×45 mm) caudal to aortic bifurcation with a saccular dilatation of infra-renal abdominal aorta (20×18×22 mm) with peripheral thrombus and fat stranding. There was tethering of adjacent small bowel loops towards the aneurysm with loss of fat planes with duodenum. A right renal calculus (7×14 mm) was additional finding [Fig.1]. 2-D echocardiography revealed grade one diastolic dysfunction, with mild tricuspid regurgitation.
Following discussion with a multi-disciplinary team of gastrointestinal surgeon, intensivists, and cardiologist an emergency extra-anatomical revascularization of lower limbs with an axillo-bifemoral graft, resection of mycotic abdominal aortic aneurysm, retroperitoneal debridement and closure of bowel defect was adjudged as the ideal treatment, in prevailing clinical and hemodynamic status of the patient. After volume resuscitation and under broad-spectrum antibiotic cover the preliminary extra-anatomical bypass was provided by an extended polytetrafluoroethylene (ePTFE) (8×55 mm) inter-ring bypass graft by an experienced vascular surgery team [Fig.2]. Through a midline laparotomy early proximal aortic control was obtained through an infra-renal clamping. Intra-operatively, the mycotic aneurysm was found extending from 1.5 cm distal to renal arteries to 1 cm proximal to aortic bifurcation. Dense peri-aortic adhesions and a primary aorto-enteric fistula between the third part of duodenum and aneurysmal sac was delineated. The mycotic infra-renal abdominal aortic aneurysm (IRAAA) was resected. After mycotic aneurysmal sac was excised, and retroperitoneal debridement was done the proximal and distal aortic stump were closed. As the duodenal defect was small it was closed primarily and omental interposition between the aortic stump and enteric contents was done [Fig.3]. The abdomen was closed over drains. As planned the patient was shifted to intensive care unit following surgery on mechanical ventilatory and high inotropic support.
Patient had a stormy post-operative period and required high ionotropic support. He was managed in consultation with cardiac care team for a post-operative cardiogenic shock. The patient recuperated well and was discharged from the hospital, with preserved lower limb vascularity and a healed abdominal wound.
Discussion
First described by Sir Astley Cooper as a ‘sometimes but serious complication of an aneurysmal aorta’, in 1829, PAEF has always offered unmatched challenges to vascular surgeons [
2]. The reported incidence of PAEF is 0.04% - 0.07% in large autopsy series and there is a limited availability of literature on the subject. Tumors, radiotherapy, and infection have been reported as the etiological factors [
3,
4]. The tethering effect of ligament of Treitz makes the third and fourth part of duodenum exposed to the pulsatile effects of aorta and eventually the commonest site for development of fistula (54%) followed by esophagus (28%) and stomach (2%). Despite the proposed mechanisms of mechanical, infectious, and inflammatory etiologies, the exact pathogenesis of PAEF is uncertain. In this case the infectious pathology of mycotic aneurysmal dilatation of the abdominal aorta was the inciting mechanism for the PAEF. Sir Astley Cooper described a classical clinical triad for PAEF which includes gastrointestinal bleeding (60-90%), abdominal pain (30-50%) and pulsatile abdominal mass (17-25%), however this triad is only seen in 11% patients [
3,
4]. Our case had two components of the triad in the form of gastrointestinal bleed and abdominal pain. Other associated symptoms comprise of back pain, fever, weight loss, easy fatiguability and sepsis [
5].
CTA with a 60% detection rate, highest of all investigative modalities, assists in revascularization planning. The findings suggestive of effacement of aortic fat planes with soft tissue thickening and tethering of adjacent bowel loops, helps in clinching the diagnosis. Extravasation of contrast from the aorta is rare [
3,
6]. In view of hemodynamic instability of our patient the other modalities like esophagogastroduodenoscopy could not be performed. Data on laboratory findings is even more meagre, with hemoglobin below 8 mmol/L and leucocytosis reported in two-thirds and one-quarter of the patients of PAEF [
3]. Our case had leucocytosis and anemia due to the ongoing sepsis and blood loss in gastrointestinal tract.
The golden management goal ascribed to PAEF is ‘to preserve life and limb salvage should be secondary’. A high index of clinical suspicion usually clinches the diagnosis. Surgery is the only interventional modality that has the probability of reducing fatal outcomes. Despite their being little consensus in the contemporary management of PAEF, timely surgical intervention plays a pivotal role in defining the outcome [
3]. Multiple management strategies are in vogue as there are many schools of thoughts [
7]. In view of gross retroperitoneal soiling, aneurysm resection, retroperitoneal toileting, omentoplasty and an extra-anatomical revascularization has been recommended and the same was offered to the patient by the authors. Endovascular repair is mostly reserved as a bridge to open surgical repair after initial resuscitation and infection control of hemodynamically stable patients [
8].
Conclusion
PAEF is a rare clinical condition with certain mortality without surgical intervention. Timely diagnosis and surgical intervention are imperative. CTA with faster scans and ubiquitous presence forms the diagnostic modality of choice. The operative dictum: save life first, limb later, formulates the guiding surgical principle. A situation tailored surgical management with multi-disciplinary team approach is necessary for a suitable outcome.
Contributors: RM: manuscript drafting, editing, patient management; VP: surgical management, critical inputs into the manuscript. RM will act as a study guarantor. Both authors approved the final version of this manuscript and are responsible for all aspects of this study.
Funding: None; Competing interests: None stated.
References
- Gelister JS, Fox JA. Primary aortoenteric fistula. J. R. Soc Med. 1987;80(7):459-460.
- Cooper A. The lectures on the principles and practice of surgery with additional notes and cases by Frederick Tyrrell. Vol. 2. London: Thomas & George Underwood, 1824.
- Milner R, Minc S. Local Complications: Aortoenteric fistula. In: Sidway AN, Perler BA ed(s). Rutherford’s Vascular Surgery and Endovascular Therapy. 9th edition. Philadelphia: Elsevier. 2019:615-623.
- Peck JJ, Eidemiler LR. Aortoenteric fistulas. Arch Surg. 1992;127(10):1191-1194.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg. 2005;92 (2):143-152.
- Chapman AH. The stomach and duodenum. In: Sutton D, Robinson PJA, Jenkins JPR et al (eds). Textbook of Radiology and Imaging. 7th ed. Elsevier.2015:575-613.
- Gordon AC, Agarwal M. Primary aorto-enteric fistula. Int J Surg Case Rep. 2016;19:60-62.
- Hassan A, Khan A, Huasen B, Banihani M. Aortoenteric fistula after endovascular mycotic aortic aneurysm exclusion: lessons learned during the COVID-19 era. BMJ Case Reports. CP 2021;14:e238875.