|
|
|
|
|
Excellent and Durable Response to Lutetium177 PSMA Therapy in a Metastatic Prostate Cancer Patient Refractory to Docetaxel Therapy: An Illustrative Case Report
|
|
|
|
Smriti Srivastava, Ajeet Kumar Gandhi, Madhup Rastogi, Rohini Khurana, Avinash Poojari, Vachaspati Kumar Mishra Department of Radiation Oncology, RML Institute of Medical Science, Lucknow, Uttar Pradesh, India. |
|
|
|
|
|
Corresponding Author:
|
|
Dr Smriti Srivastava Email: drsmritisrivastava2017@gmail.com |
|
|
|
|
|
|
|
|
Received:
19-OCT-2021 |
Accepted:
28-SEP-2022 |
Published Online:
15-OCT-2022 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Background: Prostate specific membrane antigen (PSMA) is a membrane-bound protease largely overexpressed in all prostate cancers. Differential expression of PSMA in tumor and non-tumor tissue has led to evolution of several targeted therapeutic interventions in metastatic castration resistant prostate cancer (mCRPC). Case Report: Here we present the case of 66-year-old male of advanced carcinoma prostate with multiple skeletal metastasis. He underwent bilateral orchidectomy and started on abiraterone followed by enzalutamide. Being unresponsive to both these treatments, he received 6 cycles of chemotherapy with docetaxel but had continuously rising PSA. His genomic analysis did not reveal any DNA repair defects. After performing 68Ga-PSMA-PETCT scan, he was started on 177-Lu-PSMA therapy. On receiving three cycles of Lu-177 DKFZ-PSMA-617, good clinical and biochemical response was obtained. However, on further PSA rise he was started on actinium 225-PSMA therapy. Till date three cycles of Actinium-225 PSMA therapy has been given and 68Ga-PSMA-PETCT scan demonstrated good partial response at metastatic sites with corresponding decrease in sPSA levels. The patient is now on follow up and receiving zoledronic acid at 3 months interval and is presently asymptomatic at the time of last follow up. Conclusion: PSMA directed radionuclide therapy is a promising treatment option in patients of mCRPC refractory to docetaxel therapy yielding excellent and durable biochemical control. |
|
|
|
|
|
Keywords :
|
Metastasis, Prostate Cancer, Prostate Specific Antigen, Radionuclide Therapy, Targeted Therapy.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffe8a038000000c301000001001100 6go6ckt5b5idvals|3130 6go6ckt5b5|2000F757Tab_Articles|Fulltext Castration resistant prostate cancer (CRPC) is defined as disease progression despite androgen deprivation therapy with serum testosterone at castrate levels (<50 ng/dL) and may present as one or any combination of a continuous rise in serum PSA, progression of pre-existing disease or appearance of new lesions [ 1]. Although remarkable progress in the treatment of mCRPC has been accomplished over the past years, the targeted radionuclide therapy (TRT) like Lutetium177-PSMA (177-Lu-PSMA) therapy has opened a novel perspective in management of these patients. Prostate specific membrane antigen (PSMA) is a membrane-bound protease largely over-expressed in all prostate cancers. Recent studies like the VISION trial have demonstrated the potential of 177-Lu-PSMA therapy as a viable therapeutic option in patients with metastatic prostate cancer [ 2]. Although the therapy has not been approved by FDA yet, but the results from single institutional and retrospective studies has been promising. Our case report aims to show the role of 177-Lu-PSMA therapy in a patient of mCRPC refractory to docetaxel therapy.
Case Report
A 66-year-old male with diabetes mellitus and hypertension was diagnosed with metastatic carcinoma prostate in July 2017. His Gleason’s score was 7, with a base line sPSA of 490 ng/mL, and bone scan suggestive of multiple skeletal metastasis in humerus, scapula, sternum, multiple cervical, dorsal and lumber vertebrae. He underwent bilateral orchidectomy in August 2017 and was started on monthly zoledronic acid. In the initial 1st year, he had good clinical and biochemical response on serial sPSA, done every 3 months with a nadir PSA of 1.37 ng/mL in April 2018. He had PSA progression (sPSA: 6.73 ng/mL) in October 2018 and was started on abiraterone 1000 mg once daily and prednisolone 5 mg twice daily in December 2018. But in April 2019, due to further rise in sPSA (12.82 ng/dL), abiraterone was stopped, and enzalutamide was started. After 4 months of enzalutamide therapy, the patient was found unresponsive with continuous rise in sPSA (218 ng/mL) and hence in July 2019 enzalutamide was stopped. He was then started on chemotherapy with injection docetaxel at a dose of 75 mg/m2, 3 weekly for 6 cycles from July 2019 to November 2019. He showed further biochemical, clinical and radiological progression just after one month of completion of chemotherapy (sPSA: 458 ng/mL in Dec 2019). 68Ga PSMA PET CT scan was done in December 2019 which showed diffuse PSMA expression in prostate gland, bilateral humerus, lumber vertebra and sacrum and bilateral femur; while non PSMA avid lesion were found in bilateral humerus, scapula, clavicle, sternum, all ribs and multiple vertebrae and PSMA avid multiple foci in marrow of bilateral femur [Fig.1]. His genomic analysis revealed low tumor mutation burden score with stable MSI and no mutation in germline or somatic DNA repair pathway.
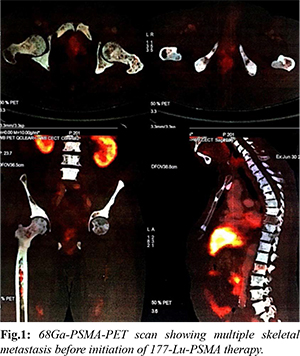
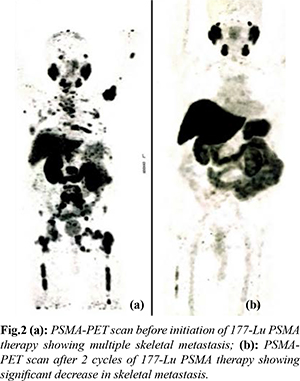
The patient was referred for 177-Lu PSMA therapy. 265 mCi of Lu-177 DKFZ-PSMA-617 by slow intravenous injection over 20 min using renal protection protocol (hydration with intravenous normal saline) under steroid cover was given. He received 2 cycles of 177-Lu PSMA therapy at an interval of 3 months, first in December 2019 and second in February 2020 respectively. He had good clinical, radiological and biochemical response [sPSA dropped from 458 ng/mL to 16.38 ng/mL in February 2020 and further 10.30 ng/mL in June 2020] and PSMA-PET scan done in June 2020 showed complete metabolic resolution of non PSMA expressing bony lesions and significantly decreased metabolic activity of PSMA expressing bony lesions and decreased activity of marrow deposit in bilateral femur [Fig.2]. He had no hematological, kidney or liver toxicity during course of treatment. He further received third dose of 177-Lu PSMA therapy in July 2020. Following 3rd dose of 177-Lu PSMA therapy his sPSA gradually started rising and reached 17.13 ng/mL in August 2020 and 29 ng/mL in October 2020. His 68Ga PSMA PET CT scan done in October 2020 showed increased PSMA avid multiple bony lesions, suggestive of progressive disease. The patient was then started on alpha PSMA therapy and 1st dose of Ac225 DKFZ-PSMA-617 was given in October 2020 at a dose of 100 KBq/kg body weight. He has received 3 doses of Actinium-225 therapy till date (last in March 2021) and recent PSMA PET-CT is suggestive of good partial response at metastatic sites with a sPSA value (July 2021) of 10 ng/mL. The patient is now on follow up and receiving zoledronic acid at 3 months interval and is presently asymptomatic at the time of last follow up.
Discussion
Current treatment options in mCRPC besides androgen deprivation therapy (ADT) incorporate newer agents like abiraterone accetate, enzalutamide and cytotoxic therapy like docetaxel. For patients resistant to the first line therapy and having DNA repair defects PARP inhibitors like olaparib and immunotherapy is considered. While Radium-223 is a treatment option given to men with bone-predominant symptomatic mCRPC having no visceral metastases [ 3]. In our patient, the treatment options including newer hormonal therapies and docetaxel therapy showed treatment failure, while he was ineligible for PARP inhibitors, immunotherapies and Radium-223 therapy. In patients who are either ineligible or resistant to these therapies, prostate specific membrane antigen (PSMA) targeting radioligand therapy is an attractive option. PSMA is a type II transmembrane glycoprotein, also known as folate hydrolase or glutamate carboxypeptidase II, which is over-expressed by the cell surface of 90-100% of all prostate cancers [ 4, 5]. The PSMA over-expression is associated with high-grade, de-differentiated, metastatic, castration-resistant prostate cancer. This differential expression of PSMA in tumor and non-tumor tissue has led to evolution of several targeted therapeutic interventions using radioligand therapy with Lutetium-177 (177Lu) and Actinium-225 (225Ac). PSMA is not entirely prostate specific and is expressed in other cells like proximal renal tubules, salivary gland and small intestine, this influences the side effect profile of PSMA targeted therapy and also on the safe radiotherapy dose which can be given to the patient [ 6]. 177Lu is a ß radiation emitter acts by binding to extracellular domain of PSMA receptor and is transported into cell by endocytosis where it causes local radiation of prostate cancer cells leading to intracellular DNA damage and apoptosis. PSMA radioligand therapy is indicated in patients of mCRPC who have failed or are ineligible for alternative cytotoxic treatment options, having adequate organ function, showing adequate radiotracer uptake on PSMA PET/CT prior to therapy. It is given at a dose of 6.0-7.5 GBq per cycle as slow intravenous injection, in 2-6 cycles at an interval of 6-8 weeks [ 7, 8] It should also be considered that not all men with mCRPC will have good response to 177-Lu-PSMA therapy. Studies also demonstrate that up to one third of patients treated show progressive disease despite of treatment. This is due to non-uniform expression of PSMA receptor by the tumor cells, this leads to low response in some sites during treatment with 177-Lu-PSMA therapy leading to rise in sPSA and disease progression. PSMA activity is evaluated by assessing activity on 68Ga-PSMA PET CT scan [ 9]. In past years several studies and meta-analysis have been published demonstrating the efficacy and safety profile of 177-Lu PSMA therapy [ 10, 11]. Review by Yadav et al. which included 16 studies demonstrated greater than 50% decline in sPSA in 46% patients while 75% patients show any PSA decline. They also reported lengthening of overall survival (OS) and progression free survival (PFS) with limited toxicity in these subsets of patients. Majority of side effects were mild and comprised hematological (23%), renal toxicity (9.5%) and xerostomia (14.5%) [ 12]. In 2021, the results of phase 3 VISION trial has established the role of 177-Lu PSMA therapy in mCRPC patients with progressive disease who had received previous treatment with one or more androgen-receptor-pathway inhibitors and one or two taxanes. This was a phase 3 trial including previously treated 831 patients of mCRPC, having PSMA-positive 68Ga labeled PSMA-11 PET CT scan. 551 patients received 177Lu-PSMA-617 (7.4 GBq every 6 weeks for four to six cycles) plus protocol-permitted standard of care and 280 patients received standard of care alone. The alternate primary end points were imaging based progression-free survival and overall survival, secondary end points were objective response, disease control, and time to symptomatic skeletal events. The addition of 177Lu-PSMA-617 resulted in improved PFS (8.7 vs 3.4 months) and OS (15.3 vs 11.3 months). They also found extension in time to symptomatic skeletal events and prolonged time to worsening of health-related quality of life and pain and delay in biochemical progression following 177-Lu-PSMA therapy. 177Lu- PSMA-617 was associated with toxic effects like hematological toxicity, renal toxicity, xerostomia, nausea vomiting and fatigue that were mainly of grade 3 or lower. This study is one of the largest randomized study on 177Lu-PSMA-617 till date and validates addition of 177Lu- PSMA-617 as a new treatment option in mCRPC. However it has certain limitations like high number of dropouts, lack of placebo control and double blind study etc [ 13]. As demonstrated in the study by Feuerecker et al., Actinium-225 therapy is a treatment option in patients resistant or intolerable to Lu-PSMA therapy, it also possess better side effect profile [ 14]. In our patient of mCRPC who was refractory to docetaxel therapy, PSMA directed radionuclide therapy yielded excellent and durable biochemical control persisting beyond 18 months and further having adequate response with alpha PSMA therapy since last 10 months. Future treatment options in these subset of patients would include re-biopsy from the prostate gland to exclude neuroendocrine histology and further lines of therapy may include docetaxel rechallenge or cabazitaxel, immunotherapies including immune checkpoint inhibitor therapies and tumor associated antigen therapies like anti-PD-L1 therapies and sipuleucel-T [ 15].
Conclusion
In patients with hormone refractory advanced mCRPC resistant to all standard cytotoxic agents. 177-Lu-PSMA therapy is an effective, safe treatment option associated with limited toxicity profile. This therapy also shows favourable efficacy with respect to imaging response and quality of life.
Contributors: SS, AKG: manuscript writing, patient management; MR, RK: manuscript editing; AP, VKM: critical inputs into the manuscript. SS will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of the study. Funding: None; Competing interests: None stated.
References - Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17:S72.
- Morris MJ, Bono JS De, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Phase III study of lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION). Available at: https://ascopubs.org/doi/abs/10.1200/JCO.2021.39.15_suppl.LBA4 Accessed on November 28, 2022.
- Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II 2020 Update: Treatment of relapsing and metastatic prostate cancer [Formula presented]. Eur Urol. 2021;79:263-282.
- Ghosh A, Heston WDW. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528-539.
- Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15:167-172.
- Ross JS, Sheehan CE, Fisher HAG, Kaufman RP, Kaur P, Gray K, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357-6362.
- Van Kalmthout LWM, van der Sar ECA, Braat AJAT, de Keizer B, Lam MGEH. Lutetium-177-PSMA therapy for prostate cancer patients-a brief overview of literature. Tijdschr Voor Urol. 2020;10:141-146.
- Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Ping Thang S, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825-833.
- Emmett L, Willowson K, Violet J, Shin J, Blanksby A, Lee J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64:52-60.
- Kim YJ, Kim Y Il. Therapeutic responses and survival effects of 177Lu-PSMA-617 radioligand therapy in metastatic castrate-resistant prostate cancer: A meta-analysis. Clin Nucl Med. 2018;43:728-734.
- von Eyben, F.E., Roviello, G., Kiljunen, T. et al. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur J Nucl Med Mol Imaging. 2018;45:496-508.
- Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand therapy with 177 Lu-PSMA for metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. AJR Am J Roentgenol. 2019;213:275-285.
- Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091-1103.
- Feuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, et al. Activity and adverse events of Actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of Lutetium-177-PSMA [Formula presented]. Eur Urol. 2021;79:343-350.
- Powers E, Karachaliou GS, Kao C, Harrison MR, Hoimes CJ, George DJ, et al. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J Hematol Oncol. 2020;13:1-13.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Srivastava S, Gandhi AJ, Rastogi M, Khurana R, Poojari A, Mishra VKExcellent and Durable Response to Lutetium177 PSMA Therapy in a Metastatic Prostate Cancer Patient Refractory to Docetaxel Therapy: An Illustrative Case Report.JCR 2022;12:98-102 |
|
Srivastava S, Gandhi AJ, Rastogi M, Khurana R, Poojari A, Mishra VKExcellent and Durable Response to Lutetium177 PSMA Therapy in a Metastatic Prostate Cancer Patient Refractory to Docetaxel Therapy: An Illustrative Case Report.JCR [serial online] 2022[cited 2026 Jan 8];12:98-102. Available from: http://www.casereports.in/articles/12/4/Excellent-and-Durable-Response-to-Lutetium177-PSMA-Therapy-in-a-Metastatic-Prostate-Cancer-Patient-Refractory-to-Docetaxel-Therapy.html |

|
|
|
|
|