|
|
|
|
|
Successful Laparoscopic Management of Acute Mesenteric Ischaemia with Gangrenous Small Bowel: World’s First Reported Case
|
|
|
|
S Islam1,2, A Maughn1,2, O Payne1,2, V Bheem1, P Harnarayan1,2 1San Fernando General Hospital, San Fernando, Trinidad and Tobago; 2Department of Clinical Surgical Science, University of the West Indies, St. Augustine, Trinidad and Tobago. |
|
|
|
|
|
Corresponding Author:
|
|
Dr. Shariful Islam Email: shar_islam7@hotmail.com |
|
|
|
|
|
|
|
|
Received:
25-JAN-2023 |
Accepted:
20-MAR-2023 |
Published Online:
25-MAY-2023 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Background: The use of laparoscopy in the management of Acute Mesenteric Ischemia (AMI) has been a topic of debate. Its application is generally limited to highly selective cases in the early stages, primarily for diagnostic purposes and potentially as a therapeutic option. In recent times, laparoscopy has emerged as an alternative to 2nd look laparotomy in an increasing number of cases. However, there remains a lack of reported cases detailing the successful laparoscopic management of AMI with gangrenous small bowel in the English literature. Case Report: We report the case of a 59-year-old male patient who presented with symptoms suggestive of AMI. A Computed Tomography (CT) scan confirmed the diagnosis, revealing gangrenous changes in the small bowel. The ischemic segment was meticulously resected using laparoscopic techniques, ensuring an adequate margin of healthy tissue. Postoperatively, the patient showed remarkable improvement. The recovery was uneventful, with no significant complications observed. Conclusion: Despite the limited recommendations for laparoscopy in AMI cases, our experience highlights the potential therapeutic utility of laparoscopy in carefully selected patients with AMI and gangrenous small bowel. |
|
|
|
|
|
Keywords :
|
Abdominal Pain, Computed Tomography, Gangrenous Small Bowel, Laparoscopy, Surgery.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1fff0173a000000460c000001000100 Introduction
Acute mesenteric ischemia (AMI) is a rare yet potentially serious condition, with an incidence ranging from 0.09% to 0.2% and accounting for less than 1 hospital admission per 1000 in the USA. Females are more commonly affected, with a ratio of 3:1. AMI is associated with high morbidity and mortality rates [ 1, 2]. The clinical presentation of patients with AMI can vary significantly. Early diagnosis and prompt therapeutic intervention are crucial in improving patient survival. Standard management options include exploratory laparotomy, damage control laparotomy, and second-look laparotomy. The role of laparoscopic surgery in the management of AMI has also been explored. However, the exact role and efficacy of laparoscopy in AMI management remain subjects of debate and controversy. The current evidence supporting the use of laparoscopy in AMI management is of low quality [ 3- 5]. Notably, successful laparoscopic management of AMI with necrotic bowel has not been documented in the English literature to date. Open conversion with damage control laparotomy remains the procedure of choice. In this paper, we present the world's first documented case of successful laparoscopic management of AMI with gangrenous small bowel and share our experience in managing this challenging case.
Case Report
A 59-year-old man presented to our accident and emergency department with five (5) days history of intermittent colicky central abdominal pain but no nausea or vomiting. The patient stated that his condition had worsened over the last 12 hours; the pain became continuous. He also claimed that he had a normal bowel action early in the morning and with no history of per rectal bleeding. The patient is a known diabetic, hypertensive and epileptic controlled on medication. The patient denied past history of post-prandial abdominal pain, thromboembolism, myocardial infarction, ischemic heart diseases, atrial fibrillation or abdominal surgery. On examination, the patient was comfortable and his mucous membranes were pink but dry. His blood pressure was mildly elevated (170/100 mm Hg) with a pulse of 104 beats per minute and her SPO2 was 98% on room air. His abdomen was mildly distended with moderate tenderness in the peri-umbilical region on palpation and there was mild guarding but no rebound tenderness. There was no palpable mass or hernia noted and the bowel sounds were absent. Digital rectal examination was normal and there was no blood on gloves. Blood investigations revealed an elevated WBC of 14×109/L with a normal hemoglobin and a mildly deranged renal function with a serum creatinine of 1.4 mg/dL but normal serum electrolytes. Chest X-ray PA view and electrocardiogram (ECG) were normal. Plain abdominal radiographs demonstrated non-specific dilated loops of small bowel [Fig.1]. An arterial blood gas analysis revealed mild metabolic acidosis. A CT scan of abdomen and pelvis with intravenous contrast revealed non enhanced segment of small bowel with fecalization [Fig.2]. Multiple filling defects were noted within the branches of SMA, approximately 10 cm distal to its origin and in the branches of right renal artery [Fig.3]. The inferior pole of right kidney was partially enhanced and there was mild free fluid in the abdomen but no free air [Fig.4].
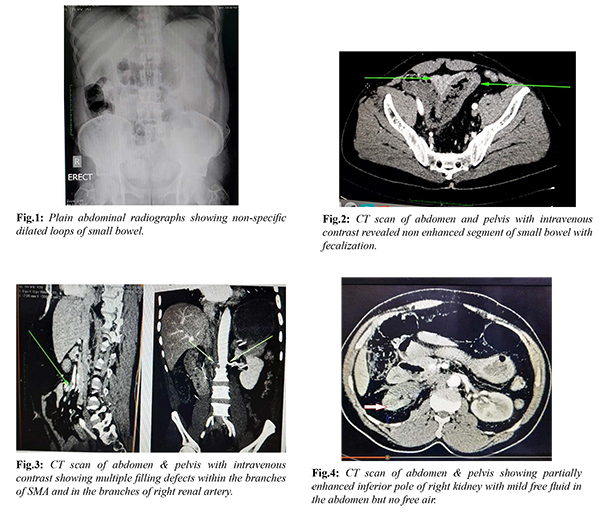
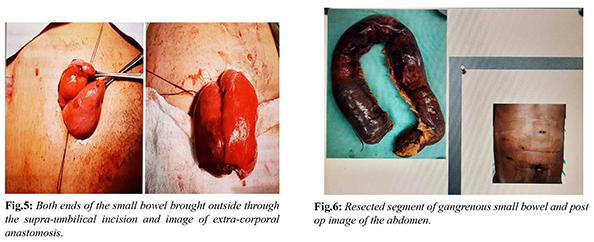
The patient was reviewed by the Medicine specialist, Cardiologist and by the Anaesthetist. A portable Echocardiogram revealed no vegetation, thrombus or any other abnormality in the heart with an EF of 68%. Broad spectrum antibiotic and intravenous heparin were started. After adequate resuscitation the patient was consented for emergency laparoscopy. This decision was made based on our past experience of successful laparoscopic management of gangrenous small bowel. However, we kept an open mind for open conversion at any point if necessary. Under endotracheal intubation with the patient in supine position, the entire abdomen was clean and draped. Pneumo-peritoneum was created by open Hasson technique via a supra-umbilical incision and maintained between 10-12 mm Hg. A gangrenous loop of small bowel with moderate blood-stained free fluids was noted on initial laparoscopy. One 12 mm port in supra-pubic area and one 5 mm port in left lower quadrant were inserted under direct vision. All blood-stained fluid was aspirated and on further exploration it was noted that approximate 50-60 cm segment of necrotic small bowel was noted in proximal ileum. However, rest of the small bowel was shinny with visible peristalsis and there were no other ischemic spots on the small intestine. The patient was hemodynamically stable; decision was made for laparoscopic resection and anastomosis. The necrotic small bowel (approximately 50-60 cm) was resected with a 45 mm endo GI stapler. The mesenteric blood vessels were slowly taken down with a harmonic scalpel. No large retrieval bag was available in the operation theatre to remove the specimen hence the resected necrotic small bowel was removed by extending the supra-umbilical incision. There was no bowel wall perforation or contamination during the entire procedure. The patient remained stable on the table; hence decision was made for primary anastomosis. Both ends of the small bowel were brought outside through the supra-umbilical incision and side to side extra-corporal anastomosis was performed with an endo GI 45 mm stapler [Fig.5]. The abdomen was thoroughly irrigated with warm normal saline and sucked out. The small bowel remains peristaltic and there is no change of colours. A drain was left in the pelvis and was brought out through the left lower quadrant port. Supra-umbilical wound was washed with saline and iodine and all the ports were closed [Fig.6]. The entire procedure was finished in two and half hours and the blood loss was very minimal.
The patient remained stable throughout the procedure; hence the anaesthetist decided to send the patient to the General Surgical ward instead of HDU or ICU. A second look laparoscopy was planned in 48 hours however; post-operatively the patient recovered exceptionally well with minimal requirement of analgesia. In the immediate post-op period, patient vitals, abdominal examination as well as arterial blood gas (ABG) were normal. The patient had started to pass flatus on day 2 hence, was allowed to have clear fluids to liquid diet and gradually advance to normal diet. The patient was discharged home on day 4 after a normal bowel action and started on long term anticoagulation therapy initially with therapeutic clexane followed by rivaroxaban. A second look laparoscopy or laparotomy was not clinically indicated in our patient. A formal ECHO cardiogram was performed and it revealed no abnormality. Serum hyper-coagulable panel assay showed no abnormality. Histology confirmed the findings of a segment ischemic small bowel. A repeat CT scan of abdomen and pelvis with intravenous contrast was done after 3 months. It revealed that the right kidney is now well perfused [Fig.7]. The patient was then switched on to aspirin and simvastatin. At two years of follow up, the patient was doing well with no further abdominal complaints.
Discussion
AMI can be either occlusive or non-occlusive. The occlusive AMI can be either embolic or thrombotic (in 67% cases) or venous thromboembolism (in 15.7% of cases), and non-occlusive mesenteric ischemia (NOMI) is usually seen in the remaining patients [ 2]. The embolic occlusive diseases patient often gives a history of recent myocardial infarction, congestive heart failure, atrial fibrillation, or peripheral arterial embolism. They usually present with a sudden onset of acute abdominal pain. On the contrary, patient with acute thrombotic occlusive diseases gives a history of chronic post-prandial abdominal pain with associated weight loss and food intolerance [ 2]. The diagnosis of AMI is very difficult as clinical markers are usually non-specific. Severe disproportionate abdominal pain or bowel emptying in a patient with risk factors for embolism, venous or arterial thrombosis and prior history of post-prandial abdominal angina; associated with certain laboratory parameters (serum lactate level: 2.1 mmol/L, pH< 7.34; WBC: 12x109 /L) could be highly suggestive of AMI [3,6]. The gold standard for the diagnosis of AMI is multi-detector CT angiography (CTA) with a sensitivity and specificity of 93.3% and 95.9% respectively [4,5,7]. However, the diagnosis of non-occlusive AMI is very challenging. CTA reports are often inconclusive. Diagnostic laparoscopy can play a vital role in the diagnosis as well as therapeutic timing in these patients and can reduce the negative laparotomy rate with its associated morbidity and mortality. The standard management of AMI with signs of SMA (superior mesenteric artery) occlusion at CTA is damage control laparotomy and in selected cases, laparotomy with resection and anastomosis, ICU admission, and then second or third look laparotomy as is necessary. Therapeutic decisions are based on the presence of peritonitis, the presence of irreversible ischemia or infarcted segments of the bowel, the general condition of the patient and the pathophysiological process underlying the ischemia [3]. The first step of the management of AMI is bowel revascularization followed by reassessment of viability of the bowel. However, a minimum of 30 minutes should be spent after re-vascularisation before making any decision about the viability of the bowel to assess the signs of adequate perfusion i.e. pulsation in mesenteric vessels, the appearance of the serosa and colour of the bowel, visible peristalsis, and bleeding from cut surfaces [2]. If an exploratory laparotomy or laparoscopy is performed as the first diagnostic step and obvious signs of bowel ischemia an on-table SMA angiography should be performed. Damage control laparotomy should be performed in absence of a skilled vascular surgeon. Once the patient is well resuscitated and stable, should be transferred to a specialized vascular centre [5]. SMA occlusion can be managed through open embolectomy or other options like hybrid procedures or endovascular stenting [2]. Percutaneous angioplasty is rarely performed due to a high risk of thromboembolism [8-10]. Thrombotic occlusions are best treated with conservative therapy involving high-dose anticoagulation and vasodilators. Venous infarction and non-occlusive MI can be managed with medical therapy and close monitoring [2]. Exploratory laparotomy is conventionally used to assess bowel necrosis, peritonitis, and other factors in SMA occlusion. However, it poses risks for critically ill patients due to the long incision. Diagnostic laparoscopy (DL) provides a less invasive alternative with 3D visualization [11-14]. In stable patients without peritonitis, DL can perform the same diagnostic functions as exploratory laparotomy. It is also useful when other imaging modalities face delays or contraindications [3]. Fluorescein has been recommended to enhance DL's sensitivity, but human studies are limited [15,16]. DL has been employed after cardiac surgery and in cases of suspicious AMI and aortic dissection, enabling earlier diagnosis and therapeutic decision-making [17-19]. It minimizes morbidity and mortality by avoiding non-therapeutic laparotomies [3]. Comparative studies show lower morbidity and blood loss with DL compared to exploratory laparotomy [17]. DL can be performed at the bedside or in the operating theatre. It is a minimally invasive procedure for diagnosis and, in select cases, therapeutics. Timely surgical intervention is crucial, as mortality rates are lower when surgery is performed within the first 24 hours [20]. DL offers benefits such as shorter operative time and reduced risks of unnecessary redo laparotomies [21]. Despite advantages, laparoscopic surgeons remain hesitant to use DL for AMI. Primary DL is feasible in highly selected cases with skilled laparoscopic and endovascular surgeons. Revascularization can be attempted via endovascular routes, and laparoscopy can evaluate intestinal perfusion. The therapeutic approach depends on patient hemodynamic status and extent of bowel ischemia. Bowel resection via laparoscopy is possible if the patient is stable with less than 50% small bowel ischemia. Unstable patients may require conversion to mini laparotomy or damage control surgery [11]. The final surgical approach, such as primary anastomosis or damage control surgery with delayed anastomosis, depends on remaining bowel condition and patient's hemodynamic status. Second-look laparoscopy is crucial after necrotic bowel resection or prior stent placement. Future studies are needed to establish DL's therapeutic role in AMI, but it can be considered for highly selected patients with skilled surgeons [1,4,22].
Conclusion
Diagnostic laparoscopy (DL) is increasingly being used as an alternative to second-look laparotomy, reducing operating time and the need for open abdomen and loss of domain. It can be performed at the bedside for ICU patients to monitor their clinical progress and reduce unnecessary laparotomies. In selected cases, DL can aid in therapeutic decision-making and timing of intervention. However, complete laparoscopic management of acute mesenteric ischemia (AMI) with resection of gangrenous bowel has not been documented. We present the world's first successful laparoscopic management of AMI with gangrenous bowel. Surgeons are encouraged to consider DL as an initial diagnostic approach in selected AMI patients before proceeding to exploratory laparotomy.
Contributors: The author SI, AM, OP, VB were responsible for drafting of the text sourcing and editing clinical images, investigation results and revising and author PH assisted in drafting, writing and critically analysing the intellectual content of the article. SI will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study. Funding: None; Competing interests: None stated.
References - OCEBM Levels of Evidence Working Group. The Oxford levels of evidence 2. Oxford Centre for Evidence-Based Medicine, Oxford. Available from: http://www.cebm.net/index.aspx?o=1025. Accessed June 12, 2023.
- Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23(1):9-20.
- Tshomba Y, Coppi G, Marone EM, Bertoglio L, Kahlberg A, Carlucci M, et al. Diagnostic laparoscopy for early detection of acute mesenteric ischaemia in patients with aortic dissection. Eur J Vasc Endovasc Surg. 2012;43(6):690-697.
- Agresta F, Ansaloni L, Baiocchi GL, Bergamini C, Campanile FC, Carlucci M, et al. Laparoscopic approach to acute abdomen from the Consensus Development Conference of the Società Italiana di Chirurgia Endoscopica e nuovetecnologie (SICE), Associazione Chirurghi Ospedalieri Italiani (ACOI), Società Italiana di Chirurgia (SIC), Società Italiana di Chirurgiad’Urgenza e del Trauma (SICUT), Società Italiana di Chirurgianell’Ospedalità Privata (SICOP), and the European Association for Endoscopic Surgery (EAES). Surg Endosc. 2012;26(8):2134-2164.
- Acosta S. Surgical management of peritonitis secondary to acute superior mesenteric artery occlusion. World J Gastroenterol. 2014;20(29):9936-9941.
- Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, et al. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World Journal of Emergency Surgery. 2017;12:38.
- Menke J. Diagnostic accuracy of multi-detector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology, 2010;256(1):93-101.
- VanDeinse WH, Zawacki JK, Phillips D. Treatment of acute mesenteric ischemia by percutaneous transluminal angioplasty. Gastroenterology. 1986;91(2):475-478.
- Klar E, Rahmanian PB, Bücker A, Hauenstein K, Jauch KW, Luther B. Acute mesenteric ischemia: a vascular emergency. Dtsch Arztebl Int. 2012;109(14):249-256.
- Block TA, Acosta S, Björck M. Endovascular and open surgery for acute occlusion of the superior mesenteric artery. J Vasc Surg. 2010;52(4):959-966.
- Islam S, Payne O, Bheem V, Harnarayan P, Dan D. Laparoscopic resection of gangrenous small bowel with acute small bowel obstruction – World’s First reported case. Innovative J Medical and Health Sciences. 2019;9(3):354-359.
- Horgan PG, Gorey TF. Operative assessment of intestinal viability. Surg Clin North Am. 1992;72(1):143-155.
- Hobson RW, Wright CB, Rich NM, Collins GJ. Assessment of colonic ischemia during aortic surgery by Doppler ultrasound. J Surg Res. 1976;20:231-235.
- Moneta GL, Lee RW, Yeager RA, Taylor LM, Porter JM. Mesenteric duplex scanning: a blinded prospective study. J Vasc Surg. 1993;17:79-86.
- Kam DM, Scheeres DE. Fluorescein-assisted laparoscopy in the identification of arterial mesenteric ischemia. Surg Endosc. 1993;7(2):75-78.
- Paral J, Ferko A, Plodr M, Raupach J, Hadzi-Nikolov D, Dolezal D, et al. Laparoscopic diagnostics of acute bowel ischemia using ultraviolet light and fluorescein dye: an experimental study. Surg Laparosc Endosc Percutan Tech. 2007;17(4):291-295.
- Bergamini C, Alemanno G, Giordano A, Pantalone D, Fontani G, Di Bella AM, et al. The role of bed-side laparoscopy in the management of acute mesenteric ischemia of recent onset in post-cardiac surgery patients admitted to ICU. Eur J Trauma Emerg Surg. 2022;48(1):87-96.
- Kim SH, Hwang HY, Kim MJ, Park KJ, Kim KB. Early laparoscopic exploration for acute mesenteric ischemia after cardiac surgery. Acute and Critical Care. 2020;35(3):213-217.
- Meriggi F, Alloni A, Gramigna P, Tramelli P, Vigano M. Acute aortic dissection with intestinal ischemia: what to do first. Ann Thorac Cardiovasc Surg. 2011;17(6):631-633.
- Jagielski M, Piatkowski J, Jackowski M. Challenges encountered during the treatment of acute mesenteric ischemia. Gastroenterology Research and Practice. 2020;5316849.
- Anadol AZ, Ersoy E, Taneri F, Tekin EH. Laparoscopic "second-look" in the management of mesenteric ischemia. Surg Laparosc Endosc Percutan Tech. 2004;14:191-193.
- JVT Tilsed, Casamassima A, Kurihara H, Mariani D, Martinez I, Pereira J, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42:253-270.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Islam S, Maughn A, Payne O, Bheem V, Harnarayan PSuccessful Laparoscopic Management of Acute Mesenteric Ischaemia with Gangrenous Small Bowel: World’s First Reported Case.JCR 2023;13:49-55 |
|
Islam S, Maughn A, Payne O, Bheem V, Harnarayan PSuccessful Laparoscopic Management of Acute Mesenteric Ischaemia with Gangrenous Small Bowel: World’s First Reported Case.JCR [serial online] 2023[cited 2026 Jan 7];13:49-55. Available from: http://www.casereports.in/articles/13/2/Successful-Laparoscopic-Management-of-Acute-Mesenteric-Ischaemia-with-Gangrenous-Small-Bowel.html |

|
|
|
|
|