6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffa442040000002e02000001000d00
6go6ckt5b5idvals|252
6go6ckt5b5idcol1|ID
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Case Report
A 51 year old lady, with a past history of atrial fibrillation and hypertension, was admitted initially for gallstone pancreatitis which settled quickly with conservative management. Incidentally, her abdominal ultrasound (USG) and further imaging with computed tomography (CT) showed a diffusely enlarged gallbladder (9.2x1.3x9.3 cm), suggestive of gallbladder cancer [Fig.1]. Thus, an inpatient cholecystectomy, usually done in the case of gallstone pancreatitis, was held off till further discussion at our multi-disciplinary meeting.
Based on the CT images, our radiologist commented that the GB mass was localised to the GB itself without any involvement of surrounding structures and distant metastases. The mass itself was well-circumscribed and avascular which is unusual for advanced GB cancer. Her tumour markers, CA19-9 and carcinoembryonic antigen were within normal range. Thus, a laparoscopy was to be done to exclude peritoneal disease before proceeding to an open cholecystectomy.
A month later, she represented acutely with severe right upper quadrant (RUQ) abdominal pain for one day and tachycardia on a background of intermittent epigastric pain since the previous admission. She had no nausea, vomiting, fevers, melena/ rectal bleeding, unintentional loss of weight or malaise. On examination, she was not hypotensive, cachexic or jaundiced. She was tender over the right upper quadrant of her abdomen but had no palpable masses. Liver function tests (LFTs) demonstrated an obstructive picture [ALP 371 (N 30-120 IU/L), GGT 370 (N 5-80 IU/L), ALT 85 (N 7-56 IU/L) total bilirubin 87 (N <20 µmol/L)] but lipase levels and white cell count were normal. She was treated with intravenous fluids and piperacillin-tazobactam (tazocin).
She then underwent a laparoscopy which was later converted to open cholecystectomy once no peritoneal seeding was visualised. A cuff of normal liver was also taken along the GB fossa. The gallbladder was enlarged and not inflamed [Fig.2]. It was also adhesive to the omentum and appeared like a benign neoplasm. We thought it may have been a gastrointestinal stromal tumour.
Pathology revealed a 160x110x100 mm gallbladder [Fig.2]. Large areas of the central tumour appeared necrotic and tissue was filled with hemorrhagic material. Histology revealed mitotically active stromal and glandular cells. Surgical margins were clear. Post-operatively, she had a low-volume bile leak due to the large raw surface area of the liver. This was managed with temporary stenting. Subsequently, her liver function tests improved and she remained pain-free. It was decided that she would be followed up with a positron emission tomography (PET) scan in 3 months time after discharge to exclude metastases, with no adjuvant chemo-radiotherapy required.
Discussion
More than 80% of gallbladder cancers are gallbladder adenocarcinomas. Gallbladder carcinosarcomas (CSGB) only accounts for less than 1% of all GB cancers [
1]. In 2012, fewer than 100 cases of CSGB have been reported in English literature [
2]. CSGB presents mostly in females between the seventh and eighth decades of life and the female to male ratio is 2:1 to 5:1 [
3].
CSGB is a very aggressive malignancy which spreads by direct invasion of adjacent organs, hematogenously and by lymph node metastasis. The poor outcome of these cancers is related to an advanced stage at diagnosis. This is due to the anatomic position of the GB and vague symptoms [
4,
5]. Although recent studies suggest some causative factors such as multistep genetic alterations, the precise mechanism of GB carcinogenesis remains unclear [
6]. Many studies have suggested the presence of cholecystolithiasis is one of the most important risk factors for CGB [
7].
The preoperative diagnosis of CSGB is difficult because imaging studies, such as, ultrasonography and CT cannot differentiate it from adenocarcinoma of GB. However, if there is speckled calcification within the tumour, ossifying carcinosarcoma can be suspected as a differential [
3]. Tumour markers such as alpha-fetoprotein, CEA and CA19-9 are also non-specific [
8]. The final diagnosis requires thorough surgical pathology studies which include immunohistological staining [
8].
CSGB demonstrates a mixture of epithelial and mesenchymatous malignant elements arising from a common cellular line. The epithelial component is most commonly represented by adenocarcinomas mostly followed by squamous cell carcinomas or a mixture of both [
8,
9]. A squamous component contributes a higher degree of malignancy [
2,
8]. The mesenchymal part varies from homogenous sarcoma (spindle cell type) to more heterogenous types like malignant bone, cartilage and smooth/skeletal muscle tissues. It is speculated that these tumours arise from totipotent stem cells, rest cells of mesoblasts that retain the capability of transformation, primitive undifferentiated mullerian stroma or paramesonephric stroma [
10]. By immunohistochemistry, the carcinomatous component is positive for epithelial markers such as cytokeratin and epithelial membrane antigen while the sarcomatoid component is positive for mesenchymal markers such as vimentin, desmin and actin [
8].
This report highlights an acute presentation of CSGB. The tumour was incidentally discovered earlier in her because of imaging for gallstone pancreatitis. Clinical symptoms of CSGB are similar to those of GB adenocarcinoma and usually relate to chronic cholecystitis or biliary colic as well as the distribution of metastases. Most patients present with intermittent right upper quadrant or epigastric pain which can be post-prandial, nausea and anorexia occurring over a few days to years [
3]. Those who present with symptoms suggestive of acute cholecystitis usually have early stage disease and a better prognosis [
11].
In the literature, a 77-year-old lady presented with a day history of severe right upper quadrant pain [
1]. The CT findings were suggestive of CGB which later tuned out to be CSGB from histology. Features more suggestive of malignant disease rather than just biliary colic are malaise and unintentional weight loss. Those who present with symptoms suggestive of acute cholecystitis usually have early stage disease and a better prognosis [
11]. The acute presentation, we propose, is possibly due to an intra-tumoral bleed or degeneration.
Therapeutic interventions have not been well defined yet. First line of management is surgical resection [
8]. Absolute contraindications to surgery for gallbladder cancer include liver metastasis, peritoneal metastases, involvement of N2 nodes (celiac, peripancreatic, periduodenal, or superior mesenteric nodes) malignant ascites, extensive involvement of the hepatoduodenal ligament, and encasement or occlusion of major vessels [
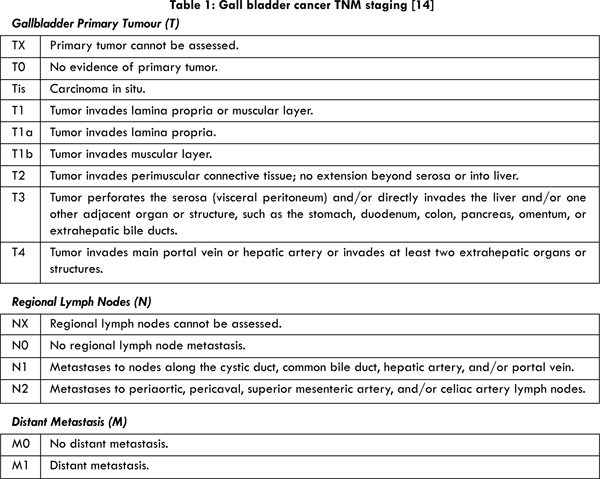
12] [Table1].
In the literature, a 70-year-old male who had CSGB without serosal invasion, was only treated surgically with no adjuvant therapy and remained healthy 54 months after. He underwent a radical cholecystectomy and hepatodudodenal ligament lymphadenectomy once it was discovered that he had a malignant mesenchymal tumour on frozen section [
6]. On the other hand, an 81 year old male, with CSGB invading the liver, underwent cholecystectomy with liver segmentectomy and lymph node dissection. However, he was later readmitted with peritoneal dissemination causing ascites and died 4 months post-operatively [
2].
Therefore, this indicates that curative treatment of CSGB without gallbladder serosal or liver invasion or lymph node involvement is possible [
6,
13]. Furthermore, the 5 year survival was 40% for patients with T2 or T3 tumours [
14] [Table 1], with a simple cholecystectomy and 90%, if a radical cholecystectomy was done [
9]. Radical cholecystectomy may involve one or more of the following: liver resection of the gallbladder fossa, common hepatic artery lymph nodes, hepatic proper artery nodes and resection of the extra-hepatic bile ducts or other organs invaded by the tumour directly [
3].
Another 59 year old lady with CSGB extending into the proximal bile ducts with no metastasis, underwent a radical cholangio-cholecystocholedochectomy. She then received 6 cycles of adjuvant chemotherapy [oxaliplatin and 5- flurouracil (5-FU)]. She remained in complete remission 6 months after chemotherapy [
8].
However, due to the rarity of CSGB and its generally poor prognosis, no optimal postoperative adjuvant therapy such as chemoradiotherapy has been established [
1,
8,
13].
CSGB appears to have a poor prognosis ranging from 2.9 to 6 months in general [
9,
15]. It can recur by metastasising to the liver, peritoneum and spread to lymph nodes. In one study, the median time to recurrence was less than a year [
1]. In particular, patients with tumours 5 cm or bigger had a significantly shorter survival than those smaller than 5 cm (mean 26.6 versus 17.7 months) [
9]. This is because more frequent local invasions and higher grade tumour may be present in tumours larger than 5 cm [
9].
Conclusion
A radical cholecystectomy may be considered even if there is no serosal invasion or if the tumour is large (5 cm or bigger). This is due to its aggressive nature. Adjuvant chemotherapy consisting of 5-FU and platinum analogs may be considered if there is evidence of more invasive disease. It is also important to note that CSGB does not always present insidiously and can do so in an acute manner due to intra-tumoral bleeding or degeneration. This may allow earlier diagnosis and perhaps improve prognosis.
References
- Park SB, Kim YH, Rho HL, Chae GB, Hong SK. Primary carcinosarcoma of the gallbladder. J Korean Surg Soc. 2012; 82:54-58.
- Kim HH, Hur YH, Jeong EH, Koh YS, Kim JC, Kim HJ, et al. Carcinosarcoma of the gallbladder: report of two cases. Surg Today. 2012;42:670-675.
- Liu KH, Yeh TS, Hwang TL, Jan YY, Chen MF. Surgical management of gallbladder sarcomatoid carcinoma. World J Gastroenterol. 2009;15:1876–1879.
- Mehrotra B. Treatment of advanced, unresectable gallbladder cancer. UptoDate. 2013. http://www.uptodate.com/contents/treatment-of-advanced-unresectable-gallbladder-cancer. Accessed on 3rd August 2013.
- Mehrotra B. Gallbladder Cancer: Epidemiology, risk factors, clinical features and diagnosis. UptoDate. 2013. http://www.uptodate.com/contents/gallbladder-cancer-epidemiology-risk-factors-clinical-features-and-diagnosis. Accessed on 3rd August 2013.
- Uzuni MA, Koksali N, Gunerhani Y, Celiki A, Gunes P. Carcinosarcoma of the Gallbladder: Report of a Case. Surg Today. 2009;39:168-171
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591.
- Pu JJ, Wu W. Gallbladder Carcinosarcoma. BMJ Case Rep. 2011 April [cited 2013 April 20]. Doi: 10.1136/bcr.05.2010.3009. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3079457/html. Accessed on 3rd Aug.2013.
- Zhang L, Chen Z, Fukuma M, Lee LY, Wu M. Prognostic significance of Race and Tumor Size in Carcinosarcoma of Gallbladder: a Meta- Analysis of 68 Cases. Int J Clin Exp Pathol. 2008;1:75-83.
- Huguet KL, Hughes CB, Hewitt WR. Gallbladder Carcinosarcoma: A case report. Journal of Gastrointestinal Surgery. 2005;9:818-821.
- Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167.
- Swanson R, Mehrotra B. Surgical management of gallbladder cancer. UptoDate. 2013. http://www.uptodate.com/contents/surgical-management-of-gallbladder-cancer. Accessed on 3rd August 2013.
- Hotta T, Tanimura H, Yokoyama S, Ura K, Yamaue H. So-called carcinosarcoma of the gallbladder; spindle cell carcinoma of the gallbladder: Report of a case. Surg Today. 2002;32:462-467.
- AJCC: Gallbladder. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp. 211-217.
- Nishihara S, Suwaki K, Moritani H, Toshimori T, Kondoh J, Oohara M, et al. Carcinosarcoma of the gallbladder. report of a case. J Bil Pancr.1990;11:635-640.