|
|
|
|
|
A Tricky Fever of Unknown Origin after Tracheal Crash
|
|
|
|
Andrea Lombi, Roberta Nicali, Cristina Izzo, Elisabetta Radin, Paola Campisi,
Piero Emilio Balbo
From the Unit of Pulmonology; Unit of Nephrology; Unit of Pathology – University Hospital “Maggiore della Carità” Corso Mazzini 18, 28100 Novara, Italy |
|
|
|
|
|
Corresponding Author:
|
|
Dr. Andrea Lombi
Email: andrea.lombi@gmail.com |
|
|
|
|
|
|
|
|
Received:
14 JUNE 2013 |
Accepted:
17-JUNE-2013 |
Published Online:
05-JUL-2013 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
A healthy, smoker farmer had a serious accident with a tracheal rupture. After 1 year of subjective wellness, he developed a persistent fever, cough and dyspnea, partially solved by antibiotics and steroids. Several investigations were performed, but they were all negative. A treatment with steroids was administered with a good initial response but subsequent worsening up to respiratory failure two years later, that required intubation and VAM. A bronchoscopy demonstrated the presence of a severe tracheobronchomalacia, possibly caused by post-traumatic chondritis. A Dumon Y silicone stent was inserted but, due to dislodgement, was replaced with a metallic stent, nevertheless a malacic progression was observed, and the patient died in two weeks due to septic shock. |
|
|
|
|
|
Keywords :
|
Tracheal Diseases, Bronchoscopy, Cough, Dyspnea, Tracheobronchomalacia, Stents.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff146003000000ec01000001000c00 6go6ckt5b5idvals|213 6go6ckt5b5idcol1|ID 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Chondritis triggered by tracheal trauma is an infrequent cause of severe tracheobronchomalacia and fever can be the main reported symptom. Large airways disease due to tracheobronchomalacia can begin in a subtle fashion which, sometimes, evolves into life-threatening disease. We report a 52 year old man with life threating tracheobronchomalacia secondary to post traumatic chondritis.
Case Report
We report a case of a 52-yr-old man, farmer, heavy smoker (35 pack/year) without major illnesses, asymptomatic for lung diseases. In April 2008, he had a serious accident with a tractor with tracheal rupture, treated with termino-terminal anastomosis, complicated by sepsis and injury to the recurrent laryngeal nerve and paresis of the left vocal cord. After a period of hospitalization in intensive care, a maxillo-facial surgery was necessary to reduce and stabilize a jaw fracture. After discharge he experienced a period of subjective wellness for 20 months. Then he reported a persistent fever (38°C) coupled with productive cough and nocturnal sweating, needing biochemical and instrumental examination.
Leukocytosis (12,000 WBC/mm3) with a preserved formula, increased inflammatory markers (ESR 80 mm/hour, CRP 10-12 mg/dL), polyclonal increase of IgA (600 mg/dL) coupled with negative autoimmunity screening panel (ANA, anti-mitochondrial antibody, Rheumatoid factor, anti-citrulline antibody, ANCA) were recorded. Furthermore, proteinuria (70 mg/dL) and microscopic hematuria in the presence of normal renal function and regular kidney echografic image were present, while Quantiferon test for tuberculosis, virological tests, sputum cultures were all negative. On the other hand, a tracheoscopic evaluation demonstrated no tracheal stenosis or other complications of the anastomosis. Chest radiograph and CT-chest demonstrated multiple intrapulmonary, hylar and high paratracheal small lymph nodes, and PET characterized them as reactive. Following hematological evaluation, bone marrow biopsy was performed and resulted normal. At this moment an empirical treatment with antibiotics was administered. Two months later, due to the recurrence of fever, a second PET positivity confirmed swollen lymph glands characterized as reactive and Angiotensin Converting Enzyme (ACE) was slightly higher than normal (65 IU/L).
Because the patient at that time refused any other diagnostic procedure (eg. fibrobronchoscopy or mediastinoscopy), we firstly hypothesized the possibility of an inflammatory disease such as sarcoidosis, that has been previously reported following trauma to the chest [ 1]. Steroid therapy with prednisone 25 mg/day was started, eventually leading to a complete remission of symptoms for 8 months. Also the nephritic sediment disappeared and IgA levels normalized (300 mg/dL), thus confirming the suggestion of a previous increase due to the local inflammation of the tracheal mucosa. The first pulmonary function test was performed and showed moderate to severe obstruction (FEV1: 37%, FVC: 101%, RV: 105%) for which a ß-2 agonist plus inhaled steroid treatment was started.
In the following months, two relapses (with fever and weight loss), were recorded following steroid therapy tapering, the first at six month after steroid reduction (prednisone from 25 to 12.5 mg/day) and the second one year later on January 2012, coupled with cough and progressive dyspnea. In both cases, the symptoms disappeared following steroid increase. At this point, we considered the possibility of a relapsing polychondritis, previously reported as a consequence of a local cartilage trauma, both as an isolated form or potentially associated to sarcoidosis or local Castelman-like lymphadenopathy [ 2].
Furthermore, in this period the patients reported a right rib cage pain, corresponding to a lytic area at X and XI ribs at chest X-ray and characterized as an ipercaptation area with PET. This bone lesion was biopsied few days later and samples showed nonspecific inflammatory infiltrates. One week later, the patient was taken to the emergency room for onset of hypoxemic respiratory failure, unresponsive to O2 therapy and CPAP, which required tracheal intubation.
Due to the difficult weaning, a bronchoscopy was performed which demonstrated the presence of expiratory collapse of the last stretch of trachea (both pars membranacea and antero-lateral walls) and main bronchi. This critical condition, previously reported [ 3], was also studied with dynamic CT. We now supposed the presence of a tracheobronchomalacia caused by post-traumatic chondritis. A Dumon Y silicone stent was placed but respiratory distress with hypercapnic respiratory acidosis appeared a few days later. A new bronchoscopy showed migration of the stent below the glottic level, with the need for removal. A covered self-expandable metallic stent was, then, placed. The intensive care hospitalization was characterized by sepsis and Acinetobacter Baumanii and Enterococcus faecium were found in blood cultures. A surgical tracheostomy was performed for impossibility of extubation but there was a continuous respiratory and hemodynamic deterioration despite maximal therapy and subsequent death occurred because of respiratory failure secondary to acute pulmonary oedema and ARDS [Table 1].
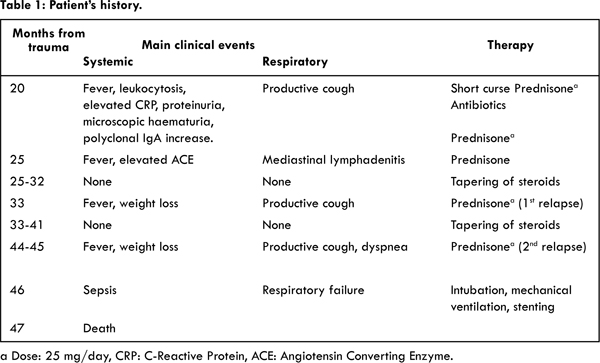
Autoptic evaluation demonstrated the presence of a diffuse inflammatory infiltrate in the tracheal and bronchial walls (chondritis), fragmentation of the cartilage, fibrosis and distrophic calcification [Fig.1]. This could account for the tracheobronchomalacia. Lungs, heart and kidneys showed a pathological pattern consistent with septic shock.
Discussion
This case underlines the difficulties in diagnosis and management of a subtle and progressive malacia of the tracheobronchial tree. It’s interesting to point out how a locally triggered disease could evolve with systemic effects, without obvious symptoms being a guide for a definitive diagnosis. This was suspected only when the disease showed characteristics of such gravity to be almost terminal. A critical review of the diagnostic steps is, therefore, necessary.
Concerning the diagnosis of sarcoidosis, we honestly can say that the diagnostic criteria were weak. The lack of histological diagnosis, albeit forced by the clinical context, was the main element. Regarding, indeed, the relapsing polychondritis, although the McAdam criteria [ 4] for diagnosis of relapsing polychondritis were not met at all, especially because of the involvement of just one cartilage site, we could consider to have dealt with a case of chondritis triggered by tracheal trauma. In this case chondritis was associated with systemic inflammation.
In fact, some cases of polychondritis showing tracheobronchomalacia alone are described [ 5- 6]. An engaging hypothesis suggests that a local cartilage trauma, with or without infection, could trigger an autoimmune autoinflammatory process directed against cryptogenic antigens, which in turn leads to inflammation of the cartilage, firstly locally and subsequently in other sites [ 7].
Some aspects delayed the correct diagnosis: the poor specificity of symptoms, the lack of involvement of other typical cartilaginous sites (ears, nose), the overlap with COPD, the presence of mediastinal lymphadenopathy. Diagnostic delay is a well-known problem and it’s estimated to be about 3 years between the first onset of symptoms and the correct diagnosis of this pathological condition [ 8].
Fever was one of the main symptoms that led the patient to evaluation. However, the lack of typical features and partial response to the combined use of antibiotic plus steroids did not allow a definite nosographic classification of the disease, requiring a series of investigations to exclude an infectious disease, an autoimmune or a hematologic disorder. The persistence of fever, especially with high temperatures, which is not typical of relapsing polychondritis, and negativity of the investigations prompted to a fever of unknown origin. Moreover, recurrent infections, furthered by the pre-existing chronic bronchitis, could play a relevant role in addition to the important systemic inflammation.
Also the early polyclonal increase of IgA associated with nephritic sediment could support the hypothesis of a systemic inflammatory trigger, started from injured tracheal mucosa. Regression of the haematological and urinary disorders after steroid therapy, even without a renal biopsy, could suggest an IgA glomerulonephritis (Berger’s disease). It is well known that IgA is thought to be an important humoral factor of the mucosal immune system (in upper and lower respiratory tract) and appears to have an antibody function working at mucosal and systemic level against various extrinsic or intrinsic substances. Glomerular IgA deposition in IgA nephropathy syndrome is thought to result from elevated levels of circulating immune complexes or aggregated IgA due to an overproduction of polymeric IgA in the serum and due to the clearance impairment of IgA immune complexes in the hepatic and splenic phagocytic system [ 9].
The correct diagnosis of tracheobronchomalacia caused by chondritis was reached too late. An earlier recognition of this severe and disabling condition could have changed the prognosis. Tracheobroncomalacia is the weakness of the walls of the front and/or side of the trachea and bronchi, due to fragmentation and loss of stability of the cartilage support [ 10]. It is a known cause of morbidity in pediatric populations and in adulthood where it can be more easily unrecognized or undiagnosed correctly. There are many causes that can lead to this condition in adults, mostly secondary to trauma (post-intubation, post external trauma to the chest, post-lung transplantation), irritant stimuli (smoke), recurrent infections (eg chronic bronchitis), chronic inflammation (relapsing polychondritis) or external compression of the trachea [ 11].
COPD and tracheobronchomalacia are not easily clinically distinguishable, symptoms are often nonspecific and overlapping and a long history of smoking is an important contribution to both diseases.
The patient was a heavy smoker but he had never been functionally evaluated before the trauma. The first pulmonary visit with complete function tests took place two years later. The flow-volume curve was suggestive of dynamic intrathoracic obstruction, although fluctuations (sometimes called “saw-tooth sign”) often found in tracheobronchomalacia were not very noticeable [ 12]. Pulmonary function tests should have been done earlier and should point to an overlapping condition other than COPD, which was considered, de facto, the main pulmonary problem. Another aspect that should have suggested a problem of the large airways was the slow but steady increase in thickness of tracheal wall and main bronchi, with the gradual onset of calcifications seen at CT chest scans [Fig. 2]. During evaluation and comparison with the previous ones, more attention was paid to the lung parenchyma instead of the large airways.
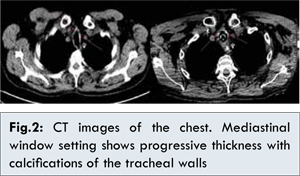
Interesting issues about treatment are present. There is no single universally accepted medical or surgical treatment for relieving symptoms and avoiding complication of chondritis with systemic manifestations. Treatment goals are multiple: to block/slow down the autoimmune process underlying the progression of disease, to maintain a patent airway and to prevent recurrent infections. Drug therapy aims to prevent or delay progression to tracheobronchomalacia, which is considered an important negative prognostic factor [ 13]. The patient, due to a late diagnosis, was treated with a too low steroid dose (25 mg/day instead of recommended 60-100 mg/day). Other immunosuppressant’s (e.g. methotrexate, cyclophosphamide etc.) were not started such as biological agents (e.g. receptor antagonists for TNF-alpha, rituximab etc.), which are frequently used in severe or resistant cases of relapsing polychondritis [ 14].
However, it is unclear whether a more aggressive drug treatment can substantially alter the natural history of the disease when it has already appeared tracheobronchomalacia. It should be pointed out, furthermore, that one of the major problems of these patients was recurrent pulmonary infection, favored by the accumulation of secretions due to limited clearance. In fact, in this case the final cause of death was a sepsis complicated with ARDS.
Airway management was complex in the later stages of disease. It is noteworthy that a relatively non-invasive maneuver as a rib biopsy was probably the precipitating factor to respiratory failure in an advanced disease. The treatment of tracheobronchomalacia encompasses the use of continuous positive pressure (C-PAP), stenting and, in more severe cases, surgical tracheobroncoplasty [ 11].
The type of stent (silicone or metallic) to be used in the first instance is still a debated question. However, the use of Dumon Y silicone stent appears appropriate and it is supported in most cases in literature. Silicone stents, deployable through a rigid bronchoscope, have several favorable properties, including stability, high tolerability, ease of removal and the relatively low cost. It has been shown that their use in patients with severe tracheobronchomalacia is associated with statistically significant improvements in dyspnea, quality of life and functional status [ 15]. However, compared to self-expandable metal stents they have a greater tendency to migrate (between 15-20% compared to 5-8% of metallic stents) [ 16], typically within the third month from implant. This event occurred also in this case, a few days after insertion, and requested the removal of the prosthesis. Metal stents appear, therefore, a second-line treatment for benign disease, even though long term benefits have been shown in patients with tracheobronchomalacia secondary to polychondritis [ 10]. This is attributable to stabilization of the airways, lower risk of migration and favorable inside/outside ratio of the diameter. A serious problem found in pathologies diffusely affecting the tracheobronchial tree is the increasingly downstream displacement of the choke point. In our case, this event led to the stent ineffectiveness, properly positioned and working. Patient ventilation and the mobilization of secretions became impossible, up to septic shock and death.
Conclusion
Post-traumatic chondritis is an infrequent cause of potentially life threating tracheobronchomalacia. Large airway disease that includes trachea and bronchi can begin in a subtle fashion which, if undiagnosed for a prolonged period of time, sometimes evolves into life threatening disease due to tracheobronchomalacia. The critical management issue is diagnosing and treating large airway disease promptly, before irreversible damage and loss of tissue integrity occurs.
References
- Pasquet F, Cottin V, Sivova N, Le Scanff J, Broussolle C, Sève P. Coexisting relapsing polychondritis and sarcoidosis: an unusual association. Rheumatol Int. 2010;30:1507-1509.
- Manganelli P, Quaini F, Olivetti G, Savini M, Pileri S. Relapsing polychondritis with Castleman-like lymphadenopathy: a case report. Clin Rheumatol. 1997;16:480-484.
- Murgu SD, Cherrison LJ, Colt HG. Respiratory failure due to expiratory central airway collapse. Respir Care. 2007;52:752-754.
- McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore) 1976;55:193-215.
- Özbay B, Dilek FH, Yalçinkaya I, Gencer M. Relapsing Polychondritis. Respiration. 1998;65:206–207.
- Tsunezuka Y, Sato H, Shimizu H. Tracheobronchial Involvement in Relapsing Polychondritis. Respiration. 2000;67:320–322.
- Cañas CA, Bonilla Abadía F. Local cartilage trauma as a pathogenic factor in autoimmunity (one hypothesis based on patients with relapsing polychondritis triggered by Cartilage Trauma). Autoimmune Dis. 2012; 2012:453698.
- Trentham DE, Le CH. Relapsing Polychondritis. Ann Intern Med.1998;129:114-122.
- Endo Y, Kanbayashi H. Etiology of IgA nephropathy syndrome. Pathol Int. 1994;44(1):1-13.
- Kalra A, Abouzgheib W, Gajera M, Palaniswamy C, Puri N, Dellinger RP. Excessive dynamic airway collapse for the internist: new nomenclature or different entity? Postgrad Med J. 2011;87:482-486.
- Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and Tracheobronchomalacia in Children and Adults An In-depth Review. Chest. 2005;127;984-1005.
- Nakajima A, Saraya T, Takata S, Ishii H, Nakazato Y, Takei H, Takizawa H, Goto H. The sawtooth sign as a clinical clue for intrathoracic central airway obstruction. BMC Research Notes. 2012;5:388.
- Sarodia BD, Dasgupta A, Mehta AC. Management of airway manifestations of relapsing polychondritis: case reports and review of literature. Chest 1999;116:1669-1675.
- Kemta Lekpa F, Kraus VB, Chevalier X. Biologics in Relapsing Polychondritis: A Literature Review. Semin Arthritis Rheum. 2012;41:712-719.
- Ernst A, Majid A, Feller-Kopman D, Boiselle P, Guerrero J, Herth F, et al. Airway Stabilization With Silicone Stents for Treating Adult Tracheobronchomalacia: A Prospective Observational Study. Chest. 2007;132:609-616.
- Chung FT, Chen HC, Chou CL, Yu CT, Kuo CH, Kuo HP, Lin SH. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. Journal of Cardiothoracic Surgery. 2011; 6:46.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Lombi A, Nicali R, Izzo C, Radin E, Campisi P, Balbo PEA Tricky Fever of Unknown Origin after Tracheal Crash.JCR 2013;3:210-216 |
|
Lombi A, Nicali R, Izzo C, Radin E, Campisi P, Balbo PEA Tricky Fever of Unknown Origin after Tracheal Crash.JCR [serial online] 2013[cited 2025 Oct 18];3:210-216. Available from: http://www.casereports.in/articles/3/2/a-tricky-fever-of-unknown-origin-after-tracheal-crash.html |

|
|
|
|
|