Introduction
Delftia acidovorans is a Gram negative bacillus normally found within the environment, and an uncommon human pathogen. We present the first reported case of Delftia acidovorans skin infection, occurring in a patient with poorly controlled type II diabetes and prominent lower limb peripheral oedema.
Case Report
Mr NP is a 70 year old pensioner from home alone, who presented to a metropolitan emergency department with a one week history of bilateral lower limb pitting oedema extending to mid-abdomen. His presentation was consistent with predominantly right sided heart failure without an obvious precipitant, and with minimal symptoms of left heart failure.
The progressive oedema was complicated by a five day history of bilateral, dull lower limb pain, decreasing mobility, erythema, pustule formation and superficial ulceration associated with extensive malodorous exudate. Mr NP describes the symptoms possibly beginning after attempting to place his shoes on his swollen feet using a shoe horn. Progressive erythema and pain over both feet occurred, with the formation of pustules that consequently ruptured, leaving superficial erosions with large amounts of malodorous exudate. Mr NP was regularly washing his wounds in the shower in the two days prior to his presentation to hospital via ambulance. Mr NP was otherwise haemodynamically stable, and describes no symptoms of rigors, symptomatic fevers or general malaise. He reports having similar episodes of lower limb oedema and pain in the past, but all were mild and self limiting.
Mr NP has a past medical history relevant for poorly controlled type II diabetes requiring insulin, eleven diagnosed acute myocardial infarctions with subsequent ischaemic cardiomyopathy and heart failure, previous aortic stenosis with mechanical aortic valve replacement, chronic obstructive pulmonary disease with a current smoking history, and recurrent deep vein thromboses and pulmonary emboli, for which he is warfarinised (additional to the aortic valve replacement). He denies any previous history of peripheral vascular disease, peripheral neuropathy, or lower limb venous disease.
Mr NP was a pensioner who lived in an outer suburban Melbourne caravan park, described by ambulance report to be in a state of squalor. Although normally independently functioning, he admitted to poor self-care and hygiene, with his health being managed in the community by a case worker.
Examination of Mr NP on presentation to the emergency department revealed an alert, dishevelled, and systemically well gentleman. Vital signs were noted as a respiratory rate of 20 breaths per minute saturating at 97% on room air, a heart rate of 57 beats per minute (regular), a blood pressure of 150/57 mmHg, and a temperature of 36.0°C. Examination of the chest found a widespread polyphonic expiratory wheeze, but was otherwise unremarkable, with no signs suggestive of left sided heart failure. The abdomen revealed pitting oedema to the level of the umbilicus, but was otherwise soft, resonant and non tender.
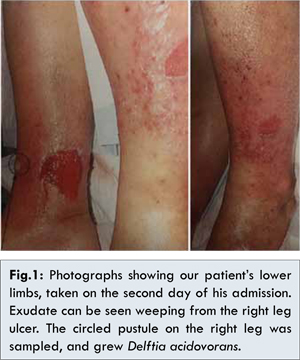
The lower limbs were oedematous bilaterally. Warmth & erythema was found bilaterally over the instep and mid-shin corresponding to areas of the aforementioned pain. Superficial erosions were present on the right medial and left lateral aspects of his legs, which were noted to be weeping clear, malodorous exudate. An unruptured pustule on the anterior aspect of the right leg was also present [Fig. 1 (taken day 2 of inpatient stay)]. Likely tinea infection was also noted between multiple toes bilaterally. Pulses were present peripherally (later confirmed by handheld Doppler), with no signs of peripheral neuropathy on neurological examination.
The relevant investigations for Mr NP revealed a leukocytosis of 18.4x109/L with predominant neutrophilia and left shift, an elevated C-reactive protein of 39 mg/L (peaking at 108 mg/L prior to directed antibiotic therapy), hyponatraemia of 132 mmol/L, and chronic renal impairment with an eGFR of 37 mL/min, marginally decreased from the previous year. Blood glucose on admission was 23.7 mmol/L without ketones, and an elevated HbA1c of 7.8%.
Mr NP was admitted to hospital for right heart failure, bilateral lower limb cellulitis & optimisation of his diabetes management. His cellulitis was empirically treated with intravenous cephazolin, additional to supportive measures of light compression, elevation and regular dressings. A wound sample was taken for microscopy, culture and sensitivities from purulent matter collected from the unruptured pustule on the right leg [Fig.1]. Microscopy revealed numerous polymorphonuclear cells without any organisms seen. Culture grew Delftia acidovorans, sensitive to ciprofloxacin and co-trimoxazole, with notable resistance to all aminoglycosides tested – gentamicin, amikacin and tobramycin – as well as amoxicillin.
In liaison with the infectious diseases service, Mr NP was commenced on a two week course of oral ciprofloxacin. A bone scan during the admission revealed no suggestions of underlying osteomyelitis. Mr NP was discharged home successfully with down-trending inflammatory markers, and clinical improvement of his heart failure, diabetes and cellulitis. He was provided with rehabilitation from allied health staff and social work arranged for increased supports in the community.
Discussion
Delftia acidovorans is a low virulence, environmental Gram negative bacillus with a wide geographical distribution, and is an uncommon clinical isolate [1]. Since its initial discovery in 1929 [2], it has been reported with gradual increasing frequency in the academic literature as a pathogenic organism, often in immunocompromised individuals [1]. To the author’s best knowledge, we describe the first reported case of D. acidovorans skin infection in the academic literature.
Delftia acidovorans has undergone numerous changes in nomenclature since its discovery, formerly being known as Pseudomonas acidovorans and Comamonas acidovorans, until being reclassified under its own new genus Delftia in 1999 [1-3]. D. acidovorans has also been of recent industrial interest, with the ability to precipitate gold nanoparticles from its surrounding environment (as a defence mechanism against oxidative damage from gold ions), resulting in the formation of gold nuggets [4]. Potential applications in the formation of bio-polyesters with D. acidovorans for use in the biomedical industry also exist [5].
Defltia acidovorans exists as straight to slightly curved Gram negative rods occurring either singularly or in pairs. Similar to the organisms it was previously grouped with (e.g. Pseudomonas), it is a strictly aerobic, motile, non-spore forming, non-lactose fermenting, oxidase & catalase positive organism. Wen et al provides an extensive microbiological description of the organism for further reference [3]. It is found throughout the environment, particularly in soil and water, which provide potential sources of exposure for patients [1,3,6].
In the previously reported literature, D. acidovorans has been shown to cause line-related bacteraemias, intravenous drug use related endocarditis, otitis externa, ocular infections such as keratitis and conjuctivitis (often in contact lens wearers), as well as a case of ventilator associated pneumonia with empyema [1,6,7]. Interestingly, these clinical manifestations are similar to those caused by Pseudomonas infections, which we postulate may be due in part to their similar microbiological phenotype, albeit with probable lesser virulence. The majority of these infections occurred in individuals immunosuppressed to some degree, however cases have been reported increasingly in immunocompetent individuals, especially related to invasive devices [1,6,7]. The tendency for D. acidovorans to form biofilm [6] likely facilitates infection in individuals with invasive devices, even amongst the immunocompetent. Additionally, these rates of infection could be expected to increase given rising rates of invasive devices used in healthcare.
There were no reports of D. acidovorans skin infection found in our literature review, and we present our patient as the first reported case. Although there is no clear history of a specific exposure, the patient was living alone in a state of squalor with poor self hygiene, either of which may have been a source of infection. Additionally, our patient was regularly washing his damaged feet in shower water, although this was after already established infection. Poorly controlled diabetes and oedematous lower limbs provide both abnormal immune function and abnormal tissue to predispose to infection.
Given that the wound culture was taken from the purulent contents of an unruptured pustule, we believe the growth to be representative of a true infecting organism. We do however note that this culture was only taken from the right leg in a patient with an apparent bilateral infection. While bilateral cellulitis is in itself an uncommon presentation, the patient has significant risk factors for developing infection in both legs. True infection is further supported by the resolution of his erythema and associated skin changes to almost normal appearing underlying skin after appropriate antibiotics. Underlying lipodermatosclerosis or other underlying dermatological conditions was thought unlikely. Whether this infection represents an initial cellulitis with pustule formation or an initial local collection with development of surrounding cellulitis was unable to be determined.
D. acidovorans infection is further supported by the clinical response after initiation of ciprofloxacin, the progressive rise in CRP while on empirical cephazolin, and its subsequent decline after initiation of ciprofloxacin. Furthermore, Pseudomonas aeruginosa is well known to cause skin and soft tissue infections in abnormal, damaged tissue such as burns. Given the similar phenotype and apparent tendency to cause similar infections, skin and soft tissue infection with D. acidovorans would not be unreasonable, especially in a predisposed individual such as our patient.
Our clinical isolate of Delftia acidovorans was sensitive to ciprofloxacin and co-trimoxazole, and resistant to amoxicillin, as well as three tested aminoglycosides - gentamicin, tobramycin and amikacin. The limited literature suggests that many isolates of Delftia acidovorans are resistant to all aminoglycosides, with particular resistance to gentamicin [1,6]. Previous reported strains were susceptible to most other anti-pseudomonal antibiotics, including broad-spectrum cephalosporins (notably also including ceftriaxone), ureidopenicillins, fluoroquinolones, carbapenems and aztreonam. All reported isolates were also sensitive to co-trimoxazole [1,6]. Importantly, Chotikanatis et al reports that some isolates variably have ß-lactamases or inducible ß-lactamases, and laboratory sensitivity testing for cephalosporins may be unreliable in vivo [1].
Conclusion
We present the first reported case of Delftia acidovorans skin infection. From a clinical perspective, many of the risk factors, clinical manifestations and treatment options are similar to that of Pseudomonal infections. A notable exception lies with the high prevalence of aminoglycoside resistance. With increasing rates of iatrogenic immunosuppression, as well as increasing use of invasive devices, this normally low virulence, environmental organism may become increasingly prevalent as an opportunistic pathogen in both immunocompromised and immunocompetent patients.
Acknowledgement:
The authors would like to thank Dr Adam Jenney for his guidance and advice.
References
- Chotikanatis K, Backer M, Rosas-Garcia G, Hammerschlag MR. Recurrent intravascular-catheter-related bacteremia caused by delftia acidovorans in a haemodialysis patient. JCM. 2011; 49(9):3418-3421.
- Tamaoka J, Ha D, Komagata K. Reclassification of pseudomonas acidovorans den doorende jong, 1926 and pseudomonas testosteronimarcus and talalay 1956 as comamonasacidovorans comb. Nov. and comamonastestosteroni comb. Nov., with an emended desc of the genus comamonas. IJSB. 1987; 37(1):52-59.
- Wen A, Fegan M, Hayward C, Chakraborty S, Sly LI. Phylogenetic relationships among members of the comamonadaceae and description of delftia acidovorans (den dooren de jong 1926 and tamaoka et al. 1987) gen. Nov. IJSB. 1999;49:567-576.
- Gwynne P. Microbiology: there’s gold in them there bugs. Nature. 2013; 495:12-13.
- Ch’ng DH, Lee W, Sudesh K. Biosynthesis and lip-asecatalysed hydrolysis of 4-hydroxybutyrate-containing polyhydroxyalkanoates from delftia acidovorans. MJM. 2012; 8(3):156-163.
- Mahmood S, Taylor KE, Overman TL, McCormicka MI. Acute infective endocarditis caused by delftia acidovorans, a rare pathogen complicating intravenous drug use. JCM. 2012; 50(11):3799-3800.
- Khan S, Sistla S, Dhodapkar R, Parija SC. Fatal delftia acidovorans infection in an immunocompetent patient with empyema. Asian Pac J Trop Biomed. 2012;2(11):923-924.