Introduction
Autoimmune thyroid disease (AITD) is the most common group of organ-specific autoimmune disorder. Pathophysiology behind the development of AITD is the loss of immunological tolerance and reactivity to thyroid autoantigens like thyroid peroxidase (TPO), thyroglobulin (TG) and thyroid stimulating hormone receptor. Infiltration of the gland by T cells and B cells that produce antibodies specific for TSHR leads to over stimulation of the thyroid follicles resulting in clinical manifestations of hyperthyroidism in Graves’ disease. AITD is often associated with autoimmune disorders involving other organs like Addison’s disease, type 1 Diabetes mellitus and pernicious anaemia.
On the other hand parathyroid nodule(s) detected sonographically can be due to parathyroid hyperplasia or adenoma. It is most often associated with hyperfunction of the parathyroid gland with hypercalcemia and raised parathyroid hormone (PTH) levels. Non-functioning parathyroid adenomas are rare. Most incidental parathyroid adenomas are found intraoperatively during thyroid surgeries [1]. Few reports of pre-operative diagnosis of normocalcemic parathyroid adenoma with normal PTH levels exist, where cystic degeneration or haemorrhage with loss of blood supply was probably the reason for loss of function [2,3].
To the best of our knowledge, this is the first report of imaging evidence of a non-functioning parathyroid adenoma with autoimmune Graves’ disease along with autoimmune haemolytic anaemia.
Case Report
A 22 year old female presented with a history of vague headache, light headedness with weight loss for the last two months. There was no history of fever, cough, diarrhoea, joint pain, liver disease, tuberculosis, and drug or alcohol abuse. Menstrual history was normal.
On examination she had pallor, tachycardia (pulse 110/minute); blood pressure was 120/80 mm Hg. There was no pigmentation or lesion in the skin or oral mucosa. She had bilateral gross proptosis with lid lag. A painless swelling in the anterior neck with tongue and finger tremor was noted. Mild splenomegaly was present.
Laboratory studies showed microcytic hypochromic anaemia with Hb: 7 gm%, RBC: 4.22x106/ mm3, Reticulocytes: 0.5%, platelets: 300x103/mm3, ESR: 18 mm/hr. White blood cells were 5000/mm3 with normal differential count. Blood glucose (fasting) was 102 mg/dL, blood urea: 18 mg/dL, creatinine: 0.5 mg/dL. Liver function tests revealed mildly increased total bilirubin 2.5 mg/dL and indirect bilirubin 1.8 mg/dL. ALT and AST enzymes were normal. Alkaline phosphatase was mildly raised 393 U/L (normal 80-290) with normal serum proteins.
Total calcium was 8.9 mg/dL (normal 9-10.5), Ionised calcium in serum 1.2 mmol/L (normal 1.12-1.32), urinary calcium excretion was 0.7 mmol/24 hours, phosphate-3.4 mg/dL (normal 2.5-4.5). Direct and indirect Coombs test for haemolytic anaemia were strongly positive indicating autoimmune haemolysis.
Serum ferritin and iron were low with raised total iron binding capacity signifying iron deficiency. Vitamin B12 was 320 pg/ml (normal 180-914). ANA and anti dsDNA were negative.
Serum free and total T4 and T3 were elevated, free T4: 3.93 ng/dL (normal 0.61-1.12), free T3: 11.95 pg/mL (normal 2.5-3.9), total T3: 2.72 ng/mL (normal 0.87-1.78), total T4: 19.26 µg/dL (normal 6.09-12.23) with low TSH: 0.07 µIU/mL (0.34-4.65). Thyroid binding globulin 154 ng/mL (normal 1.15-130) and antithyroglobulin antibodies: 36.6 IU/mL (normal 0-4.9) were raised. Serum intact parathyroid hormone was normal 59.01 pg/mL (normal 12-88). Serum cortisol, low dose dexamethasone suppression, growth hormone, LH, FSH, prolactin, insulin levels were normal.
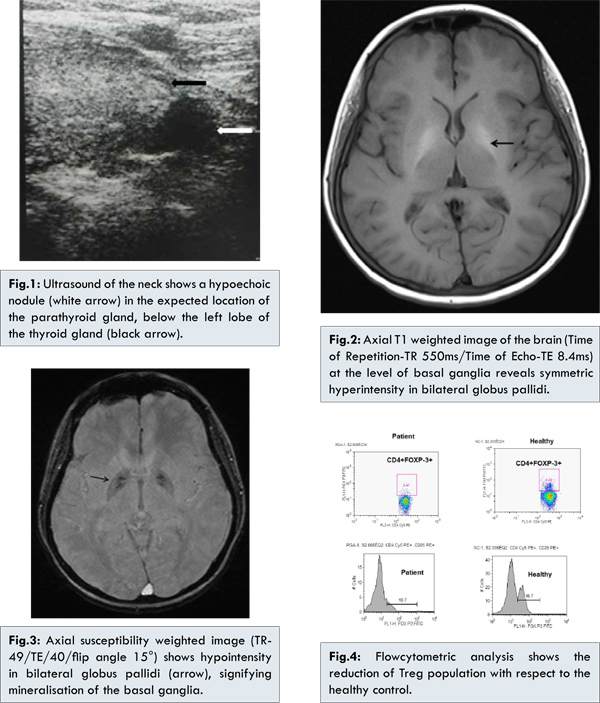
Ultrasound of the neck showed enlargement of thyroid gland. A grossly hypoechoic nodule (0.8 cm) seen just below left thyroid gland [Fig.1]. MRI of the brain showed mineralisation of bilateral globus pallidi with symmetric hyperintensities on both T1 and T2 weighted images [Fig.2] and hypointensity on susceptibility weighted (SWI) images [Fig.3]. No sellar/suprasellar lesion or enhancement was seen. To confirm the autoimmune etiologic we analysed the CD4+FOXP3+Treg cells in the peripheral blood the patient which is turned out to be 2.47 with respect to the normal control of 6.68 [Fig.4], suggesting that an autoimmune etiologic behind the disease process.
She was treated with oral iron replacement for one month to correct iron deficiency. Repeat haemogram showed increase in haemoglobin to 8.2 gm% with increase in reticulocyte count to 4.5 indicating reticulocytosis due to haemolysis. This was followed by prednisolone 1 mg/kg for one month. Thyroglobulin (115.4 IU/mL) and antithyroglobulin antibodies (4.5 IU/mL) returned to normal. Total T3 (3 ng/mL), free T3 (13.46 pg/mL) and free T4 (4.37 ng/dL) were raised. Hemoglobin increased (8.7 gm%) and reticulocyte counts decreased signifying decrease in hemolysis. Her calcium and PTH levels were normal. She was treated with tapering dose of prednisolone (10 mg/week), and carbimazole. Follow up revealed decrease in T3 and T4 levels. She was maintained on low dose carbimazole.
Discussion
This patient presented with a constellation of uncommon findings in addition to autoimmune thyrotoxicosis. She had autoimmune hemolytic anemia, a known though rare association with Grave’s disease. She also had mineralisation of deep grey matter of brain along with a parathyroid nodule in a setting of normocalcemia and normal PTH levels.
The unusual finding in the present case was the nodule in the expected location of the parathyroid gland, inferior to the left lobe of thyroid on ultrasound due to hyperplasia, adenoma or infiltration of parathyroid. It was separate from the inferior pole of thyroid and not an exophytic thyroid nodule. The second possibility is that it was a small lymph node. Facilities for sesta MIBI parathyroid scintigram were not available to confirm parathyroid origin by uptake of MIBI. However, the patient also had evidence of basal ganglia calcification on MRI. The patient did not have chronic liver disease or features of metabolic or neurodegenerative disorder. The likely cause for the basal ganglia calcification is endocrinal due derangement of calcium metabolism, either primarily due to thyrotoxicosis or secondary to disorder of the parathyroid gland [4,5].
Hyperthyroidism is known to be associated with derangement in calcium levels. While decreased calcium absorption and increased excretion of calcium due to hyperthyroid state may cause hypocalcemia, increased bone resorption may cause hypercalcemia. Both processes may counteract each other and most patients are either normocalcemic (as in our patient) or mildly hypercalcemic [6].
Disorders of parathyroid with hypoparathyroidism are also known to occur in autoimmune diseases, commonly with polyglandular autoimmune syndromes (PAS). Both PAS1 and 2 are associated with autoimmune thyroid dysfunction and hypoparathyroidism. However, our patient did not have the other manifestations of PAS, like muco-cutaneous candidiasis, pernicious anaemia, Addison’s disease, diabetes mellitus or hypopituitarism [7]. Hyperparathyroidism with a nodule on parathyroid ultrasound has also been reported with PAS by Lorraine et al. [8]. The nodule did not retain Meta-iodobenzylguanidine (MIBI), leading the authors to conclude that the nodule was a node [8]. However, increased volumes of thyroid gland may decrease the sensitivity of sesta MIBI scanning [9].
Furuto et al. reported a case of reversal of hypocalcemia to hypercalcemia in a case of chronic parathyroiditis. Parathyroidectomy revealed an enlarged parathyroid gland with lymphocytic infiltration [10]. Kifor et al. reported the presence of autoimmune hypoparathyroidism due to inhibition of PTH secretion by autoantibodies to calcium sensing receptors in two patients with Graves’ disease and Addison’s disease. In both cases, there was no immune-mediated destruction of parathyroid tissue and the second patient underwent spontaneous remission of hypoparathyroidism. The authors concluded that the intact parathyroid responds to decrease in calcium level by eventually increasing PTH secretion [11].
Spontaneous remission of autoimmune hypoparathyroidism in 2 patients has also been reported by Goswami et al, in a study involving 53 patients with sporadic idiopathic hypoparathyroidism [12]. However, sonographic appearance of the parathyroid has not been documented by either Kifor et al. or Goswami et al. [11,12].
Imbalance in the Treg population is hallmark feature of autoimmune diseases. It is postulated that the increase in the pro-inflammatory T cells or reduction in the regulatory T cells is responsible for the breakdown of self-tolerance and results in majority of the autoimmune disorders, which is also evident in our case also [13].
References
- Marchesi M, Biffoni M, Benedetti RN, Campana FP. Incidental Parathyroid Adenomas with Normocalcemia Discovered During Thyroid Operations: Report of Three Cases. Surg Today. 2001;31:996-998.
- Kiverniti E, Kazi R, Rhys-Evans P, Nippah R. Airway obstruction due to giant non-parathyroid hormone producing parathyroid adenoma. J Cancer Res Ther. 2008;4:197-199.
- Sekine O, Hozumi Y, Takemoto N, Kiyozaki H, Yamada S, Konishi F. Parathyroid Adenoma without Hyperparathyroidism. Jpn J Clin Oncol. 2004;34:155-158.
- La PH, Chen C, Liang HL, Pan HB. Hyperintense Basal Ganglia on TI-Weighted MR Imaging. Am J Roentgenol. 1999;172:109-111.
- Ho VB, Fitz CR, Chuang SH, Geyer CA. Bilateral Basal Ganglia Lesions: Pediatric Differential Considerations. Radio Graphics. 1993;13:269-292.
- Arie BR, Wysenbeek AJ, Halabe E, Blum I. Hypocalcaemia, a possible manifestation of thyrotoxicosis. Postgrad Med J. 1983;59:317-319.
- Kahaly GJ. Polyglandular autoimmune syndromes. European J Endocrinol. 2009;161:11-20.
- Pelletier-Morel L, Fabien N, Mouhoub Y, Boitard C, Larger E. Hyperparathyroidism in a Patient with Autoimmune Polyglandular Syndrome. Inter Med. 2008;47:1911-1915.
- Rink T, Schroth HJ, Holle LH, Garth H. Limited Sensitivity of Parathyroid Imaging with 99mTc-Sestamibi/123I Subtraction in an Endemic Goiter Area. J Nuclear Med. 2002;43:1175-1180.
- Furuto Kato S, Matsukura S, Ogata M. Primary hyperparathyroidism presumably caused by chronic parathyroiditis manifesting from hypocalcemia to severe hypercalcemia. Inter Med. 2005;44:60-64.
- Kifor O, Mcelduff A, Leboff MS. Activating Antibodies to the Calcium-Sensing Receptor in Two Patients with Autoimmune Hypoparathyroidism. J Clin Endocrinol Metab. 2004;89:548-556.
- Goswami R, Goel S, Tomar N, Gupta N, Lumb V, Sharma YD. Prevalence of clinical remission in patients with sporadic idiopathic hypoparathyroidism. Clin Endocrinol 2010;72:328-333.
- Christian D, Christina D, Beatrix G, Michael S. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117(3):289-300.