|
|
|
|
|
Nab-Paclitaxel in Patients with Advanced Pancreatic Cancer: Case Series
|
|
|
J B Sharma1, Palanki Satya Dattatreya2
1Consultant Medical Oncologist at Rajiv Gandhi Cancer Institute and Research Center, New Delhi; 2Senior Consultant Medical Oncologist, Hyderabad, Andhra Pradesh, India. |
|
|
|
|
|
Corresponding Author:
|
Dr. Jai Bhagwan Sharma
Email: silversword44@yahoo.com
|
|
|
|
|
|
|
|
|
Received:
23-JUN-2014 |
Accepted:
16-JUL-2014 |
Published Online:
10-AUG-2014 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Introduction: Pancreatic cancer is one of the top ten causes of cancer related deaths world-wide. The only treatment offering an advantage in terms of overall survival is radical complete resection of pancreatic carcinoma. But, only 10-20% present with resectable disease at the time of diagnosis. Single agent gemcitabine still remains the standard therapy for advanced/ metastatic pancreatic cancer after indecisive conclusions with combination therapy of oxiplatin and gemicitabine. Nab-paclitaxel (albumin bound paclitaxel) has demonstrated a better overall survival in phase III trials in combination with gemcitabine. Case Report: The two cases mentioned below are cases of advanced pancreatic cancer which have demonstrated an affirmative response to combination therapy of nab-paclitaxel and gemcitabine in first line and beyond. Conclusion: Gemcitabine, although the standard of care in the treatment of advanced pancreatic cancer, shows better efficacy and survival when used in combination with nab-paclitaxel. |
|
|
|
|
|
Keywords :
|
Paclitaxel, Deoxycytidine, Pancreatic Neoplasms, Oxiplatin, Humans.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffa8e8050000001703000001000100 6go6ckt5b5idvals|351 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States and eighth worldwide [1]. Pancreatic adenocarcinoma is one of the commonest exocrine pancreatic malignancy that accounts for more than 80% of the malignant neoplasm of the pancreas [2]. The incidence of pancreatic cancer correlates with increasing age with a peak incidence of the disease occurring in the 65–75 year age group [3].
Treatment of pancreatic cancer patients remains one of the major challenges of oncologists, despite the advances in cancer therapy. Till date, the radical surgical resection of pancreatic carcinoma remains the only treatment offering an advantage in terms of overall survival with 5-year survival of 15-25%. Unfortunately, only 10-20% of patients present with resectable disease at the time of diagnosis [4]. Initially single-agent gemcitabine was the standard therapy for advanced/metastatic pancreatic cancer, improving medial survival of 5.65 months and offering a significant clinical benefit compared with fluorouracil [5]. Combination of gemcitabine and oxiplatin failed to improve overall survival (OS) in a recently presented Eastern Cooperative Oncology Group trial [6]. Nab-paclitaxel (Albumin-bound paclitaxel particles for injectable suspension is a cremophor-free, 130-nanometer particle form of paclitaxel) in combination with gemcitabine has demonstrated a potential to increase drug delivery to tumors in pre-clinical studies [7,8]. This effect translated into a better overall survival in the phase I/II studies, in which nab-paclitaxel was used in combination with gemcitabine for patients of advanced pancreatic disease [9]. Here are the two cases in which nab-paclitaxel is used with gemcitabine which has displayed an affirmative response.
Case Reports
Case 1
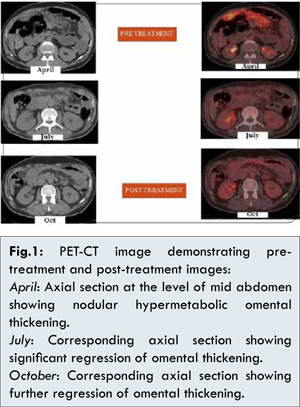
A fifty one years old male patient came with history of abdominal distension and umbilical hernia with no significant past history. Clinical examination revealed presence of ascites. Initial investigations followed by contrast enhanced CT (CECT) abdomen revealed cystic lesion approximately 8.3×6.5 cm size at pancreatic tail with mild fluidic peritoneal cavity. Whole body PET-CT displayed a hypermetabolic bilateral lung nodules, diffuse omental and peritoneal thickening along with nodular peritoneal deposits. Umbilical nodule biopsy revealed metastatic adenocarcinoma. Final diagnosis was made as adenocarcinoma tail of pancreas with omental and lung metastasis with TNM staging T2N1M1. Exploratory laparotomy and anterior abdominal wall defect closure was done under general anesthesia. Tumor antigen CEA level on 26 April 2012 was <0.50 ng/mL; CA 19.9 was 5.8 unit/mL; CA 72.4 on 2nd May 2012 was 9.08 unit/mL. Immunohistochemistry (IHC) tests (CK7+, EMA+, CDX2-, CK20-, TTF-1-) of the lesions revealed adenocarcinoma of gastrointestinal origin. Following the surgery the patient was treated with injection gemcitabine 1000 mg/m2 and injection nab-paclitaxel 100 mg/m2 on days 1,8,15 (q4weekly) and supportive therapy of G-CSF 300 mcg for two days starting 24 hours after chemotherapy. The patient tolerated the therapy well and there was regression in the omental thickness seen by the end of the 6th cycle of chemotherapy which was evident in the PET-CT scan done in October [Fig.1]. During the 7th cycle, patient developed incisional hernia which as repaired with laparotomy. During exploration no lesions were found in pancreas, there was no ascites and the anterior abdominal wall resected margins were negative for malignancy.
A fifty seven year old diabetic lady presented with left upper abdominal pain, high fever along with nausea and vomiting. Initial investigation of hematological parameter was within normal limits. Ultrasonography of abdomen showed heterogeneous mass in the pancreatic tail region-probably space occupying lesion (SOL) or adherent bowel loops. Contrast enhanced CT scan revealed lesion in the pancreatic tail with infiltrative SOL measuring 4×5 cm with splenic vein encasement and multiple perisplenic collaterals, also found were multiple hypo-attenuating SOL in liver suggestive of metastasis. Whole body PET-CT scan was done which revealed metabolically active disease in the pancreatic tail encasing splenic vessels with loco-regional lymphadenopathy suggestive of malignancy. Ultrasound guided fine needle aspiration cytology (FNAC) of the lesion was suggestive of papillary adenocarcinoma. CT-guided trucut biopsy confirmed papillary adenocarcinoma of pancreas. Patient was examined for CA 19-9 [3.38 U/mL] and carcinoma embryonic antigen (CEA) [0.5 ng/mL], both the parameters were within normal limit.
She was put on four cycles of intravenous (IV) gemcitabine (1800 mg) on day 1 and oxiplatin (180 mg) on day 2. Follow-up investigations after the chemotherapy displayed a partial metabolic response to the treatment but also the appearance of a new lesion in the anterior abdominal wall. The treatment plan was changed to six cycles of intravenous nab-paclitaxel
(200 mg) and carboplatin (150 mg). PET-CT done after the completion of chemotherapy revealed non-FDG avid primary in pancreatic tail with both FDG and non-avid secondaries in liver. There was focal hypodense lesion in the tail of pancreas with peripheral rim of calcification suggestive of chronic pancreatitis. She was advised to follow-up after three months.
First follow up after completion of chemotherapy revealed normal hematological parameters, serum CEA and CA19-9. X-ray showed a calcified oval area in the left paravetebral location opposite L1-L3 vertebrae. There was no evidence of disease progression.
Discussion
Most cases of pancreatic cancer present in advanced stage, the disease therapeutic options are also minimized hence prognosis is generally poor. Systemic therapy is used in advanced cases with a primary intent of palliation and increased survival. Immunohistochemistry (IHC) becomes a very significant diagnostic aid when the ambiguity arises about ascertaining the primary lesion in a multiple metastatic scenario. In the first case discussed, the IHC markers fit in as mentioned in the diagnostic tree by Dennis et al. [10].
The PRODIGE trial, evaluated the FOLFIRINOX regimen against single agent gemcitabine for patients with metastatic pancreatic cancer with good performance status. It demonstrated a statistically significant increase in progression free survival and median overall survival in the group receiving FOLFORINOX, but the grade ¾ toxicities reported was also higher in this group [11].
Single-agent gemcitabine administered at the dose of 1,000 mg/m2 on a weekly 30 minute infusion basis is more active in terms of palliative effects and survival than 5FU, and as such, it is considered the standard treatment in advanced pancreatic adenocarcinoma [5]. It has been observed that weekly schedule of gemcitabine 1,500 mg/m2 at a constant infusion rate achieved a response rate of only 9.1%, with a median PFS of 3.5 months and a median OS of 6.1 months. In comparison, the results of the efficacy and tolerance assessments of the gemcitabine and oxiplatin combination are better than those of single agent gemcitabine administered as a ?xed-dose infusion [8].
Nab-paclitaxel consists of particles of paclitaxel in the nanometre-size range, stabilized with human albumin. The use of albumin as a vehicle eliminates the solvent-related toxicities and obviates the need for steroid and antihistamine premedication. Furthermore, albumin has the potential to increase drug delivery to tumors by initiating albumin receptor (gp60)-mediated transcytosis across endothelial cells [7] and accumulating drug in tumors due to binding of albumin to secreted protein, acidic and rich in cysteine (SPARC) [9].
SPARC plays a key dynamic role in the process that leads to increased cancer aggressiveness [12]. SPARC over expression has displayed better response to nab-paclitaxel in a phase I/II study by Von Hoff et al. [9] with gemcitabine plus nab- paclitaxel in patients with advanced pancreatic cancer. In the first case, use of gemcitabine and nab-paclitaxel combination has achieved excellent complete response without any significant toxicity. In the second case, nab-paclitaxel and carboplatin emphasized the usefulness of this combination in comparison to the gemcitabine and oxiplatin combination.
Nab-paclitaxel and gemcitabine has tolerable adverse effects with substantial anti-tumor activity and can be considered in the treatment of advanced pancreatic carcinoma. This has been demonstrated in the phase II study [9] and has been further supported in the phase III MPACT study. The overall survival (8.5 month Vs. 6.7 month) and 1 year progression free survival (16% Vs. 9%) in the MPACT study was significantly higher in the nab-paclitaxel and gemcitabine arm. The most common grade 3 toxicities were neutropenia and reversible neuropathy [13]. In comparison to these studies, the two presented cases were put on different dosage of nab-paclitaxel. In the second case, nab-paclitaxel was used in varied combination with other chemotherapeutic regimens as a second line therapy. Even with these variations the response achieved is very encouraging. These responses reassure us to try nab-paclitaxel in different combinations and also even in the later settings to enhance the disease response and improve the survival in patients presenting with progressive and advanced stages of pancreatic cancer.
Conclusion
The two case reports mentioned an affirmative response to treatment with nab-paclitaxel in combination with gemcitabine in patients with advanced pancreatic cancer. However, both the patients are currently in the follow up stage.
References
- Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB 2008;10:58-62.
- Alexakis N, Halloran C, Raraty M Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. Br J Surg 2004;91:1410-1427.
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2006;20:197-209.
- Loos M, Kleeff J, Friess H, Buchler MW. Surgical treatment of pancreatic cancer. Ann N Y Acad Sci 2008;1138:169-180.
- Burris HA, Moore MJ, Anderson J Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 1997;15:2403-2413.
- Poplin E, Levy DE, Berlin J, Rothenberg ML, Hochster H, Mitchell E, et al. Phase III trial of gemcitabine (30-minute infusion) versus gemcitabine (fixed-dose-rate infusion [FDR]) versus gemcitabine-oxaliplatin (GEMOX) in patients with advanced pancreatic cancer (E6201). J Clin Oncol 2006;24:180s.
- John TA, Vogel SM, Tiruppathi C, Malik AB, Minshall RD. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol 2003;284:L187-196.
- Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, et al. Nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov 2012;2:OF1-10.
- Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-4554.
- Dennis JL, Hvidsten TR, Wit EC, Komorowski J, Bell AK, Downie I, et al. Markers of adenocarcinoma characteristic of the site of origin: Development of a diagnostic algorithm. Clin Cancer Res 2005;11:3766-3772.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;64:1817-1825.
- Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-signaling system. Mol Biol Cell 2003;14:3977–3988.
- Von Hoff DD, Ervin TJ, Arena FP, Chiorean EG, Jeffrey RJ. Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT). Clin Oncol 2012;30:(suppl 34; abstr LBA148).
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Sharma J B , Dattatreya PSNab-Paclitaxel in Patients with Advanced Pancreatic Cancer: Case Series.JCR 2014;4:295-299 |
|
Sharma J B , Dattatreya PSNab-Paclitaxel in Patients with Advanced Pancreatic Cancer: Case Series.JCR [serial online] 2014[cited 2025 Dec 18];4:295-299. Available from: http://www.casereports.in/articles/4/2/Nab-Paclitaxel-in-Patients-with-Advanced-Pancreatic-Cancer.html |

|
|
|
|
|