Introduction
Liver cancer is the second most common cause of cancer related mortalities with very poor prognosis (mortality to incidence ratio of 0.95) and approximately 746,000 deaths, as reported in GLOBOCON 2012 [1]. Hepatocellular carcinoma (HCC) is asymptomatic in early stages, and almost 85% of patients are diagnosed at intermediate or advanced stage. Patients with advanced HCC are either ineligible to, or have poor prognosis and risk of recurrence with conventional care. Even for patients undergoing surgical resection, recurrence rates may be as high as 50% after 2 years and 76% by 10 years [2].
The increased overall survival by sorafenib in phase III advanced HCC study provided a new therapeutic option as well as stimulated research to unfold new signalling pathways involved in HCC. Consequently, sorafenib got approved as the first systemic agent for advanced HCC. However, poor tolerability and response confine the application of sorafenib in advance HCC management [3].
PI3K/AKT/mTOR signalling pathway has a vital role in cell proliferation, migration, survival and angiogenesis and can prove to be an encouraging target. Dysregulation in mTOR signalling pathway is evidently associated with 48% of HCC as well as poor prognosis. Everolimus, the mTOR inhibitor has been reported to improve the overall survival in metastatic renal carcinoma and subependymal giant cell astrocytoma. Even clinical data from HCC patients showed that everolimus was well tolerated, with moderate antitumor efficacy in HCC patients [4].
Everolimus is the orally active mTOR inhibitor approved in anticancer applications and could be a potential option for advanced HCC treatment [3,5]. Here we report a case of successful cessation of advanced, unresectable HCC progression with the continued therapy of everolimus.
Case Report
In December 2010, a 65 year old female presented with a 3 weeks history of abdominal pain in right hypochondriac region and loss of appetite. The patient had no prior or family history of any malignancy. There was no history of smoking, alcohol consumption or blood transfusion. Patient had a history of bronchial asthma. On physical examination, the conjunctiva was pale, sclera was not icteric and no lymphadenopathy was detectable in axillary and inguinal regions. Lungs were clear, and there was no abnormal heart sounds on auscultation. Per abdomen examination revealed a soft abdomen with mild tenderness in right hypochondriac region. Initial ultrasound showed mild hepatomegaly with multiple well defined heterogenous hyperechoic solid mass lesions with tiny cystic areas within and hypoechoic peripheral rim around it. The largest lesion noted was of 7.5 x 5.5 cm in antero-inferior segment of right lobe suggestive of multiple liver metastases. The ultrasound findings were confirmed with CECT abdomen which revealed hepatomegaly with multiple, varying size, hypodense, mildly enhancing lesions in both lobes of liver, largest in the inferior part of right lobe, measuring 8.6 x 6.0 cm suggesting possibility of multiple metastases from occult primary cancer. Portal vein was normal [Fig.1].
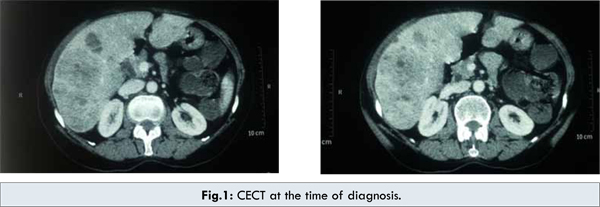
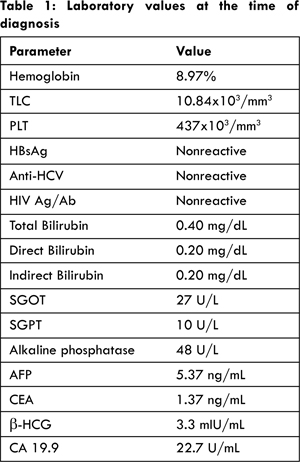
Laboratory data demonstrated anemia, normal liver function tests and no evidence of past or existing hepatitis B, hepatitis C or HIV infection. No abnormality was detected in chest X-ray. Alfa fetoprotein (AFP), carcinoembryonic antigen (CEA) and ß-HCG levels were normal. Table 1 provides the values of laboratory panel and tumor markers conducted at the time of diagnosis. Cytological examination of the lesion revealed sheets of acini and singly scattered round to polygonal cells displaying mild anisokaryosis with moderate cytoplasm. The findings were positive for HCC. Patient underwent CT guided biopsy of the liver mass which showed liver tissue with a tumor arranged in nests, sinusoids and trabeculae of malignant hepatocytes displaying pleomorphism and hyperchromatism with intranuclear and cytoplasmic vacuolation; confirming the diagnosis as grade I HCC. Due to advanced stage of disease, with Barcelona Clinic Liver Cancer (BCLC) stage B, Child-Pugh class B and TNM stage T2N0M0, the patient was considered inoperable.
In view of unresectable lesions, the patient was initiated on oral chemotherapy with sorafenib 400 mg twice daily for one month. The patient could not tolerate sorafenib as she developed skin rashes, bullous eruptions, hand and foot syndrome, and it was discontinued. Subsequently, intravenous chemotherapy was started with adriamycin 50 mg and cisplatin 80 mg (spread over two days). After 3 cycles of adriamycin and cisplatin based intravenous chemotherapy, CT abdomen demonstrated regression in size and number of lesions with the largest lesion now measuring 6.3 x 7.4 cm. Chest X-ray at this stage demonstrated two nodular opacities in right lower zone likely to be old healed granulomas. Intravenous chemotherapy was continued for total 6 cycles; however, it resulted in significant gastrointestinal and bone marrow toxicity, thus had to be discontinued. CT scan after 6th cycle showed multifocal HCC with no significant change as compared to previous scan suggesting a stable disease.
Due to intolerance to IV chemotherapy, oral chemotherapy was started with capecitabine 1000 mg twice daily (for 2 weeks followed by off for 1 week). After 3 weeks the capecitabine dose was increased to 2250 mg/day but it failed to elicit any clinical response in 6 weeks. In July 2011, it was decided to switch the patient to oral evertor (everolimus) 10 mg once daily for two months. After 2 months the dose was reduced to 5 mg once daily. The patient underwent multiple follow up CT scans during the entire treatment duration; the latest CT was performed in October 2012, which demonstrated stable disease with no increase in number or size of the cancerous nodules since the time of diagnosis [Fig.2]. The liver enzymes and the AFP remained normal throughout the course of the treatment [Fig. 3,4]. The patient was followed till December 2012 and there has been no radiological or clinical progression of her disease with continued evertor 5 mg once daily therapy.
Discussion
HCC management is complex due to asymptomatic nature of disease, presence of underlying conditions and poor prognosis, especially in advanced stages. Most of the patients are diagnosed in intermediate or advanced stages where conventional therapies not only have restricted application but also have poor outcome. Therefore, sorafenib, the first FDA-approved systemic therapy is treatment of choice for advanced HCC ineligible to surgical resection or liver transplantation. However, sorafenib have an alarming safety profile and might have poor response in some patients [6].
Fatigue (54%), dermal toxicity (45%), and hypophosphatemia (36%) were the most frequent side effects documented with sorafenib in a liver transplantation HCC study. Moreover, sorafenib was withdrawn because of intolerance or side-effects in 36% patients while dose was reduced in 91% patients on sorafenib therapy [7]. Hand-foot skin reactions (HFSR) are most common complications of sorafenib which typically develop in the first few weeks of therapy. A meta-analysis reported 33.8% incidence of HFSR in phase II and III trials across solid tumours with sorafenib. Asian population appears to be more susceptible to sorafenib toxicity demonstrating about 40% and 82% incidence of HFSR in two isolated phase III trials conducted on Asian population [2]. Parallel results were obtained in present case where the sorafenib therapy was withdrawn due to the occurrence of HFSR.
Laboratory data have implicated the mTOR signalling pathway to various oncogenic processes, including cell survival and angiogenesis. Chronic activation of mTORC1 was found to be associated with hepatocyte damage and development of HCC in a mouse model [8]. Moreover, mTOR signalling was found to be associated with poor prognosis in HCC patients [9]. Hence, mTOR inhibition is an interesting target for anti-cancer therapies. Serolimus, an mTOR inhibitor demonstrated limited efficacy in a phase II trial which evaluated 23 treatment naïve patients with advanced HCC. One patient each had an overall and partial response while the 8 patients had stable disease [3].
Everolimus, the only oral, mTOR inhibitor has established efficacy in advanced renal cell carcinoma, advanced neuroendocrine tumours and hormone receptor positive/human epidermal growth factor receptor-2 negative advanced breast cancer [3]. mTOR inhibition with everolimus, both in vitro (Human HCC cell lines) and in vivo (murine xenograft model) was documented to reduce tumor growth and increase survival. In mouse, everolimus (5 mg/kg) treatment showed a significant reduction in tumor growth (1039 mm3 vs. 2396 mm3; p < 0.05) as well as increased median overall survival (19 days vs. 16 days, p < 0.05), both compared with placebo [9]. Encouragingly, everolimus was reported to have a better safety profile in a phase I/II trial including 28 patients with advanced HCC. 10 mg/day everolimus was well tolerated and no dose-limiting toxicities were experienced [3]. A phase I study which experimented with both daily (n=21) and weekly (n=18) dosing schedule of everolimus, concluded that 7.5 mg/day of everolimus is maximum tolerable dose however, we started with 10 mg/day and down titrated to 5 mg/day post 2 months. In that study, one patient exhibited partial response and 21 patients had stable disease (daily dose, 71%; weekly dose, 44%). Median overall survival was 7.7 months (95% CI, 2.1–13.3 months, with daily dose) and 5.7 months (95% CI, 0–12.3 months, with weekly dose) [3].
Considering growing line of evidences supporting use of everolimus in HCC, everolimus for Liver Cancer Evaluation (EVOLVE-1), a phase III study was conducted in patients with advanced HCC and unresponsive or intolerant to sorafenib treatment. Completed in February 2014, the EVOLVE-1 concluded that everolimus did not improve overall survival in study population. Median overall survival with everolimus and placebo was 7.56 and 7.33 months, respectively (HR 1.05; 95% CI 0.86-1.27; P = 0.675). Anemia (7.8%), asthenia (7.8%), anorexia (6.1%), and increase or reappearance of hepatitis B virus (6.1%) were the most common grade 3/4 adverse reactions reported with everolimus [10]. However, in our case the patient tolerated everolimus well which inhibited the disease progression effectively. Till December 2012, there has been no radiological or clinical progression of her disease while she was still on evertor 5 mg/day.
Conclusion
This is the first reported case from India where we used everolimus for 17 months in patient with advanced, unresectable HCC. Everolimus therapy was started in our patient since she could not tolerate sorafenib and IV chemotherapy while oral capecitabine was ineffective. Last follow up assessment with CT scans show a stable disease as per modified WHO criteria. Evidences though favour everolimus use in HCC, lack unanimity across clinical studies. More clinical case reports can help guide the evolving use of everolimus in advanced HCC.
Acknowledgments: The author wishes to thank Dr Prashanth, Dr Aravind & Ms Deepika for providing editorial & technical assistance in the development of this article
References
- Fact Sheets by Cancer [Internet]. [cited 2014 Mar 26]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed on June 3rd 2014.
- Frenette C, Gish R. Targeted systemic therapies for hepatocellular carcinoma: clinical perspectives, challenges and implications. World J Gastroenterol WJG. 2012;18(6):498-506.
- Finn RS. Current and Future Treatment Strategies for Patients with Advanced Hepatocellular Carcinoma: Role of mTOR Inhibition. Liver Cancer. 2012;1(3-4):247-256.
- Grabinski N, Ewald F, Hofmann BT, Staufer K, Schumacher U, Nashan B, et al. Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol Cancer. 2012;11:85.
- Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepatic Med Evid Res. 2012;4:19-37.
- Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig Dis Sci. 2012;57(5):1122-1129.
- Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, et al. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25(2):180-186.
- Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5(217):ra24.
- Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135(6):1972–1983, 1983.e1–11.
- Zhu AX, Kudo M, Assenat E, Cattan S, Kang Y-K, Lim HY, et al. EVOLVE-1: Phase 3 study of everolimus for advanced HCC that progressed during or after sorafenib. J Clin Oncol [Internet]. 2014 [cited 2014 Mar 24];32(suppl 3; abstr 172). Available from: http://meetinglibrary.asco.org/content/123157-143