Introduction
Preterm infants are among the most heavily transfused of patient groups. Modern transfusion support of neonates requires specialized or modified blood components to these patients who are often immunocompromised and/or affected by very complex medical and surgical illnesses. We will present in this case report the clinical scenario that oriented us to the diagnosis of a “Transfusion-associated graft-versus-host disease (TA-GVHD)” which is a rare complication of transfusion of cellular blood components producing a graft-versus-host clinical picture with concomitant bone marrow aplasia. The disease is fulminant and rapidly fatal in the majority of patients. TA-GVHD is caused by transfused blood-derived, alloreactive T lymphocytes that attack host tissue, including bone marrow with resultant bone marrow failure.
Case Report
A 29 week preterm neonate was born by caesarean section because of preterm active labour and rupture of membrane (PPROM), to a 20 year-old primigravidae mother. The mother had a smooth pregnancy course until the current hospitalization. The birth weight of baby was 1020 grams. He had poor respiratory effort at birth, was stabilized, intubated, received positive pressure ventilation and then transferred to the neonatal intensive care unit where he was attached to nasal synchronized intermittent positive pressure ventilation (NSPPV) for 4 days.
On the 12th day of life, he had central line associated blood stream sepsis due to gram negative bacilli, extended spectrum beta lactamase producer (Klebsiella pneumoniae, ESBL). This was managed by umbilical venous catheter removal, intravenous meropenem for 7 days, and insertion of a new central venous catheter (CVC) line. Two days after discontinuation of antibiotic, he developed recurrent apnea unresponsive to intravenous caffeine. Investigations revealed no signs of new infection but significant anemia with a hemoglobin of 7.3 g/L.
In view of his emerging clinical conditions, we decided to transfuse him with fresh, leukocyte reduced; matched 15 cc packed red blood cells over 3 hours. Blood transfusion was uneventful however, on the second day, he had fine diffuse maculopapular skin rashes that disappeared after few hours [Fig.1], to reappear and persist again the second day with signs of severe sepsis, dusky appearance, hypoperfusion, respiratory failure, hyperglycemia, but the most remarkable findings was the significant skin dryness with peeling and the development of a skin necrosis and scaring on the left leg [Fig.2]. Despite all the supportive efforts made to save him, with mechanical ventilation, fluid infusion and inotropic agents, wide spectrum antibiotics, we lost him. To be noted the abnormality of his complete blood count that showed pancytopenia included leukopenia to 900 cells/mL and thrombocytopenia to 30,000/dL, there was increase of C-reactive protein (CRP), normal level of ALT, and negative results of cultures taken from blood and cerebral spinal fluid.
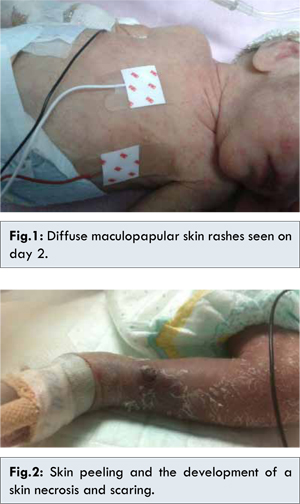
This critical scenario including: extreme prematurity, very low birth weight, history of previous antibiotics use, blood transfusion, the skin findings especially the necrosis, and the pancytopenia lead us to the clinical diagnosis of a transfusion associated graft versus host disease (TA-GVHD) in a preterm baby after PRBs transfusion from a non-relative donor.
Discussion
Allogenic red blood cell (RBC) transfusion remains an important supportive and life-saving intervention for neonatal intensive care patients. In neonates, apart from concerns about transfusion-associated infections, they present specific clinical and immunologic characteristics that require selected blood component products; leukocyte-reduced, gamma-irradiated, and ABO-Rh-compatible RBC products are used for transfusions in neonates, especially those whose birth weights are less than 1,200 grams [1].
Graft vs. host disease (GVHD) in extremely low birth weight neonates following blood transfusions is probably more frequent than is generally thought [2]. This disease was reported in humans in 1959 after allogeneic bone marrow transplantation (BMT). TA-GVHD is associated with marrow aplasia and therefore has a more rapid and fulminant course, nearly always resulting in the death of the patient [3,4].
Pre-term babies have a more immature immune system than those born at term. Their underlying immaturity is further compromised by other factors such as transfusions, surgery and nutritional status that may affect their immune status [5]. The risk of TA-GVHD is very small in the preterm infants who are transfused from random blood donors [5,6]. All cellular blood products, including red cells, platelet and granulocyte concentrates, and even fresh plasma, contain viable, immunocompetent T lymphocytes. All of these products have been implicated in TA-GVHD [4]. The donor cells are rejected if the recipient is not immune suppressed. However, in the presence of an underlying immune deficiency, either congenital or secondary to chemotherapy and/or radiotherapy, the recipient cannot reject the foreign T cells, which proliferate resulting in a GVH reaction and the clinical picture of TA-GVHD. Presence of a “one-way HLA match between donor and recipient” is associated with a significantly increased risk of TAGVHD [7].
Different transfusion practices may also play a role in the reported increased prevalence of TA-GVHD in some countries like Japan compared with other countries such as the US. In Japan, fresh blood which may be ‘warm’, that is, never refrigerated or less ?24 hour old, as well as directed donations were commonly used [6]. Mincheff has shown that, after 2 weeks of storage, leucocytes progressively undergo apoptosis and fail to stimulate and respond in a mixed leucocyte culture [8]. The critical number of lymphocytes required to induce a graft-versus-host reaction appears to be 1x107 lymphocytes per kilogram of body weight. Estimates of the viable lymphocytes counts in commonly used blood products are as follows: platelets: 4x107 lymphocytes per single donor unit; leukocytes: 5-10x109 lymphocytes per unit; and erythrocytes: 1x109 lymphocytes per unit of whole blood. So, a single transfusion of 10 ml/kg may supply a sufficient number of lymphocytes to initiate engraftment for a neonate [9].
In neonates, the clinical picture is similar to that seen in adults; however, the onset is delayed [4]. A constellation of clinical features related to skin, gastrointestinal tract, liver and the bone marrow, in an appropriate setting, must arouse suspicion of TA-GVHD. A lower threshold for performing skin biopsy aids in supporting the diagnosis [10]; but the diagnosis is missed more easily in neonates than in adults. Skin rashes are very common for other reasons, especially in premature infants, occurring in 9-12% of them. Similarly, the long median time interval (4 weeks) between transfusion and clinical signs of TA-GVHD delays or even prevents consideration of the diagnosis, because the clinical manifestations of TA-GVHD are attributed to the underlying illness as septicemia, viral infection, drug reaction or to prematurity [4]. Unfortunately, the rapid clinical deterioration of affected patients offers limited opportunity for premorbid diagnosis [9].
In our preterm baby, with constellation of symptoms within few days of blood transfusion with rapid deterioration of his clinical status in 24 hours that disabled us to do skin or bone marrow biopsy to complete the evidence, but our suspicion was highly enough to make a diagnosis otherwise of neonatal sepsis, especially when we performed full sepsis work-up with negative results, so the clinical condition, essentially the skin findings and the alarming laboratory results of pancytopenia and bone marrow failure should lead us to think positively about transfusion associated graft versus host disease (TA-GVHD) and always keep it in our differential diagnosis of lethal neonatal diseases, despite working in non favored condition like that of Lebanon, with no reported similar cases previously.
Attempts at treatment of TA-GVHD are largely ineffective. In contrast with GVHD post BMT, these patients do not respond to corticosteroids, antithymocyte globulin, cyclosporine and/or growth factors [9]. However, there are reports in the literature of spontaneous resolution of the disease [11]; the rapid and fulminant onset of TA-GVHD associated with pancytopenia and resultant overwhelming infections contribute to the high mortality seen in TA-GVHD [12].
Prevention of TA-GVHD is of paramount importance as it cannot be treated successfully. Patients at risk must be identified and transfused with irradiated cellular blood products, as irradiation inhibits proliferation of donor lymphocytes and thereby their initiation of GVHD, The American Association of Blood Banks (AABB) recommends a minimum dose of 2500 cGy at the centre of the irradiation field with a minimum dose of 1500 cGy at any point in the field, if correctly delivered, should be adequate to prevent TA-GVHD [13]. Although a blanket policy employing gamma-irradiated RBC units for all infants cannot be justified purely on a scientific basis, legal and logistical factors have strongly favoured such practice. Thus, current practice suggests that it is reasonable to gamma-irradiate cellular blood components for all infants during their first postnatal year, depending on the spectrum of infants being managed at individual hospitals [14].
Conclusion
TA-GVHD should be considered in the differential diagnosis of transfused preterm infants with sepsis accompanied by skin rashes, pancytopenia, gastrointestinal and liver dysfunction. Prevention through reduction of unnecessary blood transfusion, using nonrelatives’ donors, leukocytes reduced blood and blood irradiation is the only effective treatment.
References
- Nunes dos Santos AM, Trindade CEP. Red blood cell transfusions in the neonate. Neoreviews. 2011;12(1):e13-19.
- Flidel O, Barak Y, Lifschitz-Mercer B, Frumkin A, Mogilner BM. Graft versus host disease in extremely low birth weight neonate. Pediatrics. 1992;89(4 Pt 1):689-690.
- Mathé, G. Essai de traitement de sujets atteints de leucemie aigue en remission par irradiation totale suivie de transfusion de moelle osseuse homologue. Revues Francaises d’Etudes Clinique et Biologique. 1959;4:675-704.
- Schroeder ML. Transfusion-associated graft-versus-host disease. British Journal of Haematology. 2002;117:275-287.
- Strauss RG. Data-driven blood banking practices for neonatal RBC transfusions. Transfusion. 2000;40:1528-1540.
- Ohto H, Anderson KC. Posttransfusion graft-versus-host disease in Japanese newborns. Transfusion.1996;36:117-123.
- Gokhale SG, Gokhale SS. Transfusion-associated graft versus host disease (TAGVHD) - with reference to neonatal period. J Maternal Fetal Neonatal Med. 2014;1:1-5.
- Mincheff M. Changes in donor leukocytes during blood storage. Implications on post-transfusion immunomodulation and transfusion-associated GVHD. VoxSanguinis. 1998;74:189-200.
- Sanders MR, Graeber JE. Posttransfusion graft-versus-host disease in infancy. Journal of Pediatrics. 1990;117(1):159-163.
- Gupta A, Bansal D, Dass R, Das A. Transfusion Associated Graft Versus Host disease, Indian Pediatrics. 2004;41:1260-1264.
- Mori S, Matsushita H, Ozaki K. Spontaneous resolution of transfusion-associated graft-versus-host disease. Transfusion. 1995;35:431-435.
- Brubaker DB. Transfusion-associated graft-versus-host disease. Human Pathology. 1986;17:1085-1088.
- Menitove JE. Standards for Blood Banks and Transfusion Services, 19th ed., American Association of Blood Banks, Bethesda: 2000;20.
- Widness JA. Treatment and Prevention of Neonatal Anemia. NeoReviews. 2008;9:e526-e533.