|
|
|
|
|
Fatal Hepatic Necrosis after Transcatheter Arterial Embolization for Blunt Hepatic Trauma
|
|
|
Masafumi Toguchi1, Tsuyoshi Tajima1, Akiyoshi Hagiwara2, Toru Igari3, Kanehiro Hasuo1
Departments of Radiology1, Emergency2 and Pathology3, National Center for Global Health and Medicine, Tokyo-162-8655, Japan. |
|
|
|
|
|
Corresponding Author:
|
Dr. Tsuyoshi Tajima
Email: ttajima@kyj.biglobe.ne.jp
|
|
|
|
|
|
|
|
|
Received:
04-OCT-2014 |
Accepted:
31-DEC-2014 |
Published Online:
15-JAN-2015 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Hepatic necrosis after transcatheter arterial embolization (TAE) is rare because of the dual blood supply to the hepatic parenchyma via the portal vein and hepatic artery. We describe a patient with blunt hepatic injury who had a portal-systemic venous shunt in the right posterior segment of the liver and who suffered a fatal hepatic necrosis after TAE. A 74-year-old female with blunt hepatic injury was treated by TAE: both the right hepatic artery and middle hepatic artery were embolized using gelatin sponge particles. She developed multiple organ failure and died four days after the TAE. Autopsy revealed massive necrosis in the right lobe and left medial segment of the liver. To the best of our knowledge, this is the first report of hepatic necrosis after TAE for blunt liver trauma related to a portal-systemic venous shunt. |
|
|
|
|
|
Keywords :
|
Embolization, Hepatic Artery, Portal Vein, Portasystemic Shunt, Liver, Autopsy, Humans.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffd807060000004403000001000100 6go6ckt5b5idvals|416 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Transcatheter arterial embolization (TAE) for hemodynamically stable patients with blunt hepatic trauma has become a safe and standard therapeutic procedure, and is widely performed [1-5]. Hepatic infarction after TAE is unusual because of the dual blood supply to the hepatic parenchyma via the portal vein and hepatic artery.
We report a patient who suffered a fatal hepatic necrosis immediately after hepatic TAE. The necrosis is thought to have been related to the patient’s portal-systemic venous shunt. To the best of our knowledge, this is the first report of fatal hepatic necrosis related to a portal-systemic venous shunt as a complication of TAE using gelatin sponge particles.
Case report
A 74-year-old female was brought to our hospital by ambulance 9 days after falling about 1 meter to the ground in her house. Her chief complaint was lumbago. On admission, her blood pressure was 98/54 mmHg, her heart rate was 108 beats per minute and her respiratory rate was 18 breaths per minute, with a SpO2 of 93%. She was alert and hemodynamically stable. Serum chemistry showed mild hepatic and renal dysfunction, aspartate aminotransferase 63 IU/L, alanine aminotransferase 91 IU/L, serum creatinine 3.29 mg/dL, and blood urea nitrogen 135 mg/dL. Unenhanced computed tomography (CT) of the abdomen showed no evidence of liver injury or hemoperitoneum, except for a compression fracture of the second lumbar spine.
The patient was treated conservatively. Her condition was uneventful except for low grade fever and mild liver dysfunction on the sixth day. However, on the seventh day, she complained of progressive abdominal pain, and showed hypotension and tachycardia. Under a diagnosis of delayed peritoneal hemorrhage, contrast-enhanced CT of the abdomen was performed, and showed a laceration in the right anterior and left medial segments of the liver, hemoperitoneum and extravasation of contrast media [Fig.1]. In addition, a prominent portal-systemic venous shunt was detected in the right upper posterior segment of the liver [Fig. 1C-E].
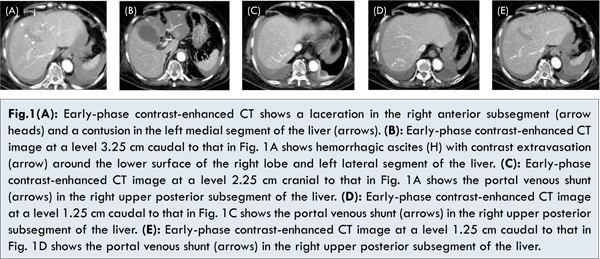
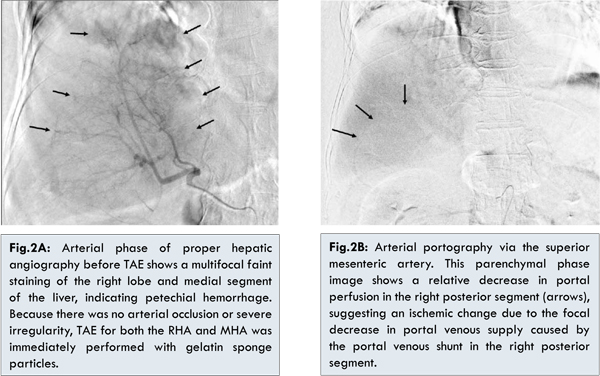
Under a diagnosis of hepatic laceration with arterial bleeding, TAE was immediately performed. The arterial phase of proper hepatic angiography before TAE showed multifocal faint staining in the right lobe and medial segment of the liver, indicating petechial hemorrhage [Fig.2A]. Arterial bleeding from the laceration in the right anterior and left medial segments was suspected. Arterial portography via the superior mesenteric artery showed that the main trunk and bilateral branches of the portal vein were patent, but it also showed a relative decrease in portal perfusion in the right posterior segment [Fig.2B]. Although a focal decrease in portal venous supply caused by the portal venous shunt was suggested in the right posterior segment, in order to stabilize hemodynamic status, TAE was immediately performed for both the right hepatic artery (RHA) and middle hepatic artery (MHA) using gelatin sponge particles (Gelpart; Nippon Kayaku, Tokyo, Japan). Celiac arteriography after embolization showed stasis of the peripheral branches of the RHA and MHA, while a patent left hepatic artery (LHA) arising from the left gastric artery was confirmed. The patient’s systolic blood pressure rose to 116 mmHg after embolization from 87 mmHg before TAE.
After TAE, the patient developed severe liver dysfuncion following multiple organ failure and her general condition deteriorated; she died 4 days after TAE in spite of medical treatment. Autopsy revealed swelling of the entire right lobe [Fig.3] and pathologic specimens showed massive necrosis in the right lobe and left medial segment of the liver. In the inferior surface of the medial segment of left lobe, laceration and hematoma were found.
Discussion
TAE is an essential treatment procedure in the management of blunt hepatic injury and has been universally adopted for patients who are hemodynamically stable [1-5]. Its safety is attributed to the dual blood supply to the hepatic parenchyma via the portal vein and hepatic artery.
Generally, in cases of hepatocellular carcinoma, major portal vein obstruction is a contraindication for transcatheter arterial chemoembolization (TACE) because of the risk of hepatic infarction or abscess formation [6]. Liver infarction is reported to occur in 6% of patients with hepatic tumors who undergo TAE [6].
There have been some well-organized reports on the incidence of hepatic infarction after TAE for trauma [4,5,7-9]. In the report with the largest number of the cases [5], 30 of 71 (42.3%) patients who had a high-grade liver injury developed hepatic infarction after TAE; 2 of those 30 died of hepatic infarction. In other reports, 1 of 14 (7.1%) patients [4], 3 of 23 (13.0%) patients [7], 3 of 23 (13.0%) patients [8], and 7 of 31 (22.6%) patients [9] developed hepatic infarction. There was one fatality in each of these reports. There may be differences between cases in the degree of hepatic infarction that occurred after TAE.
With respect to hepatic necrosis after TAE, there have been a few reports that mention embolized arterial branches or embolic materials. Misselbeck et al. [9] report that 6 patients developed hepatic necrosis after TAE. Five of these 6 underwent embolization of the LHA or RHA or their branches and none of the patients who were selectively treated in this way died, however, the other patient, who underwent nonselective embolization of the proper hepatic artery (PHA) using gelatin sponge particles, died. Letoublon et al. [7] report that 3 of 23 patients developed hepatic necrosis after TAE. One among three patients underwent TAE in the superior posterior branch of the RHA, the other one in the posterior branch of the RHA, and the remaining one patient in the main trunk of RHA. Only one patient who underwent TAE in the posterior branch of the RHA had the portal venous injury.
In our case, the most significant reason for the fatal hepatic necrosis of both the right lobe and medial segment of the left lobe was thought to be a decrease in the relatively large amount of arterial flow by TAE for the PHA under portal hypo-perfusion by the portal-systemic venous shunt in the right hepatic vein. Cho and Lunderquist [10] report that in the cirrhotic liver, the so-called peribiliary plexus (PBP) is markedly developed compared to that in the normal liver. Although our case was not complicated with liver cirrhosis, a compensatory arterial blood supply via the PBP would have existed but might have been insufficient because of the extensive and wide embolized area.
To the best of our knowledge, few cases of hepatic necrosis related to a portal-systemic venous shunt have been reported. It is important to recognize that a portal-systemic venous shunt causes hypoperfusion of the liver and could cause or contribute to a fatal hepatic necrosis after TAE regardless of the degree of the shunt.
Conclusion
TAE is a useful treatment option for cases with blunt liver trauma but caution must be exercised to prevent a severe hepatic infarction in TAE for patients with a hepatic injury complicated by a portal venous shunt.
References
- Hagiwara A, Yukioka T, Ohta S, et al. Nonsurgical treatment of patients with blunt hepatic injury: effi cacy of transcatheter arterial embolization. Am J Roentgenol. 1997;169:1151-1156.
- Croce MA, Fabian TC, Menke PG, Waddle-Smith L, Minard G, Kudsk KA, et al. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Annals of Surgery. 1995;221:744-755.
- Ochiai T, Igari K, Yagi M, Ito H, Kumagai Y, Iida M, et al. Treatment strategy for blunt hepatic trauma: analysis of 183 consecutive cases. Hepato-Gastroenterology. 2011;58:1312-1315.
- Monnin V, Sengel C, Thony F, Bricault I, Voirin D, Letoublon C, et al. Place of arterial embolization in severe blunt hepatic trauma: a multidisciplinary approach. Cardiovasc Intervent Radiol. 2008;31:875-882.
- Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. J Trauma. 2009;66:621-629.
- Sakamoto I, Aso N, Nagaoki K, Matsuoka Y., Uetani M., Ashizawa K. et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605-619.
- Letoublon C, Morra I, Chen Y, Monnin, Voirin D, Arvieux C. Hepatic arterial embolization in the management of blunt hepatic trauma: indications and complications. J Trauma. 2011;70:1032-1037.
- Saltzherr TP, van der Vies CH, van Lienden KP, et al. Improved outcome in the non-operative management of liver injuries. HPB. 2011;13:350-355.
- Misselbeck TS, Teicher EJ, Cipolle MD, Pasquale MD, Shah KT, Dangleben DA, et al. Hepatic angioembolization in trauma patients: indications and complications. J Trauma. 2009;67:769-773.
- Cho KJ, Lunderquist A. The peribiliary vascular plexus: the microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology. 1998;147:357-364.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Toguchi M, Tajima T, Hagiwara A, Igari T, Hasuo KFatal Hepatic Necrosis after Transcatheter Arterial Embolization for Blunt Hepatic Trauma.JCR 2015;5:19-23 |
|
Toguchi M, Tajima T, Hagiwara A, Igari T, Hasuo KFatal Hepatic Necrosis after Transcatheter Arterial Embolization for Blunt Hepatic Trauma.JCR [serial online] 2015[cited 2025 Oct 21];5:19-23. Available from: http://www.casereports.in/articles/5/1/Fatal-Hepatic-Necrosis-after-Transcatheter-Arterial-Embolization-for-Blunt-Hepatic-Trauma.html |

|
|
|
|
|