|
|
|
|
|
Nab-Paclitaxel in Recurrent Ovarian Cancer: An Institution Based Retrospective Study
|
|
|
Rajesh Kumar Singh, Sangeeta Pankaj, Vamsi Raj Kota, Sumit Kumar
Department of Radiation Oncology, Regional Cancer Centre, Indira Gandhi Institute of Medical Sciences, Sheikhpura, Patna, Bihar, India. |
|
|
|
|
|
Corresponding Author:
|
Dr. Rajesh Kumar Singh
Email: drprashanthp@gmail.com
|
|
|
|
|
|
|
|
|
Received:
13-OCT-2014 |
Accepted:
14-JAN-2015 |
Published Online:
28-FEB-2015 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Introduction: Nab-paclitaxel is a novel cremophor free nanoparticle of albumin-stabilized paclitaxel. We evaluated the efficacy and toxicity of the nab-paclitaxel in recurrent ovarian cancer patients. Methods: 17 patients of recurrent epithelial ovarian cancer with platinum and taxane resistance defined by persistent or progressive disease following recurrence within six months of treatment completion and measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) or elevated CA-125 (= 70 U/mL) in patients without measurable disease were included in trial. Patients were treated with nab-paclitaxel for six cycles or until disease progression. Results: Median age of patients was 62 years; 76% of patients had stage IIIC or IV disease, 82% had Eastern Cooperative Oncology Group performance status of 0, and 88% had prior surgery. For assessable patients, the objective response rate (ORR) was 58% (6 complete responses [CR] and 4 partial responses [PR] among 17 assessable patients). In patients evaluated with RECIST only, the ORR was 44.4% (one CR and three PR of 9 patients). In patients with only elevated CA-125, ORR was 75% (5 CRs and 1 PRs of 8 patients). Median time to response was 1.3 months (range 0.5 to 4.8 months). Estimated median progression-free survival was 8.5 months. The most frequent grade 3 to 4 treatment-related toxicities were neutropenia (35%) and neuropathy (11%). Conclusion: Nab-paclitaxel as a single agent in patients with recurrent epithelial ovarian cancer seems to be well tolerated and effective in patients who are previously treated with paclitaxel or platins. |
|
|
|
|
|
Keywords :
|
Paclitaxel, Epithelial Neoplasms, Neutropenia, Disease-Free Survival, Taxoids, Humans.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff8417060000007303000001000600 6go6ckt5b5idvals|434 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Ovarian cancer is the 5th leading cause of death from gynecologic cancer [1]. Because of a lack of specific symptoms or effective screening, the majority of women with epithelial ovarian cancer (EOC) have stage III or IV disease at initial diagnosis. After initial cytoreductive surgery, the treatment of advanced epithelial ovarian cancer includes platinum-based chemotherapy. First-line therapy generally includes intravenous platinum (carboplatin or cisplatin) and taxane (paclitaxel or docetaxel) [2,3]. 50% of patients experience relapse by 36 months (5-year survival <20%). In relapses that occur more than 6 months after completion of prior platinum based chemotherapy (platinum-sensitive disease), re-treatment with platinum is associated with response in 40-72% [4-6]. A novel albumin–bound paclitaxel (nab-paclitaxel) offers advantages over standard-formula paclitaxel or docetaxel administration, may be an effective strategy as monotherapy in patients with platinum/taxane-sensitive disease [7-9]. In this study, we sought to determine the response rate and feasibility with taxane based on therapy using nab-paclitaxel. The objectives of this study were to determine the response rate to treatment with nab-paclitaxel in patients with epithelial ovarian cancer, evaluation of progression-free survival (PFS), overall survival, during treatment, and the safety and toxicity of the treatment. The efficacy of the treatment was determined primarily by the overall response rate (ORR), based on the assessable population who received study drug.
Materials and Methods
Study design: This was an institutional based retrospective study for patients treated with single-agent nab-paclitaxel between Jan 2012 to March 2014.
Patients & Methods: 17 patients were enrolled and inclusion criteria consisted of patients more than 18 years of age having histologically or cytologically confirmed epithelial ovarian cancer with Eastern Cooperative Oncology Group performance status of 0 to 2, disease meeting international criteria proposed by the Response Evaluation Criteria in Solid Tumors (RECIST) Committee or CA-125 greater than 2 times the upper limit of normal (> 70 U/mL) in the absence of measurable disease, received prior platinum-based chemotherapy, adequate hematologic, renal and hepatic function, treatment-free interval more than 6 months since completion of platinum-based chemotherapy.
Patients were excluded if they had previously untreated stage I, grade 1 disease, more than one prior chemotherapy regimen or any prior non-platinum regimen, taxane within 6 months of registration, prior radiation or nab-paclitaxel, non-epithelial disease, non-measurable disease with CA-125 <70, hypersensitivity to paclitaxel, evidence of CNS involvement and serious uncontrolled medical or psychiatric illness, another malignancy within the last 5 years, chemotherapy-naïve disease and pregnant or nursing mothers.
Treatment: Eligible patients enrolled onto this study were administered 260 mg/m2 of nab-paclitaxel intravenously over 30 minutes on day 1 every 3 weeks for six cycles. Patients with complete response (CR) received an additional two cycles, for a maximum of eight cycles. Patients who developed disease progression or intolerable toxicity while on study were taken off treatment. Verification of inclusion and exclusion criteria, a pregnancy test (physical examination, assessment of Eastern Cooperative Oncology Group performance status, assessment of peripheral neuropathy, disease assessment (computed tomography or magnetic resonance imaging), CA-125, CBC count with differential and platelet count, and laboratory tests (total bilirubin, serum creatinine, AST, ALT, alkaline phosphatase, serum calcium) were analyzed. Assessment of disease status (eg: computed tomography, magnetic resonance imaging, x-rays, and so on) was performed every 9 weeks; CA-125 was measured with every cycle. Recommended follow-up, after treatment completion, was at 3-month intervals, up to 18 months (measured from the start of treatment), to collect response and survival data.
Assessing Response and Toxicity: RECIST criteria version 1.1 was used to evaluate response and progression. Only changes in the tumor lesion’s largest diameter (unidimensional measurement) was used, as per RECIST. Analysis of serum CA-125 was conducted. Assessment of response by CA-125 was based on normalization for more than 28 days from the baseline value (CR), a sustained more than 50% reduction from the baseline value (partial response [PR]), a sustained less than 50% increase in CA-125 over 28 days in the absence of any new clinically measurable disease (stable disease [SD]), or a sustained more than 50% increase in CA-125 or development of new clinically measurable disease, measured from the nadir during treatment (progressive disease [PD]). Adverse events were recorded throughout the trial. Common Terminology Criteria for Adverse Events version 4.0 was used to grade toxicities. Each event’s relationship to treatment was assessed by the treating physician and documented. Additional information for each event, such as treatment required, eventual outcome, and therapy delay or dose reductions, were also collected. Adverse events were recorded for up to 30 days after the last study treatment.
Statistical Analysis: ORR was analyzed using descriptive methods (frequency counts and proportions of response [CR plus PR]) and is presented with its 95% CIs. For patients who achieved a major objective response (CR or PR), the median and range of both the time to response and the duration of response (DoR) were measured and calculated. Patient baseline characteristics and disease factors were summarized using descriptive statistics. For the safety population (all patients who received at least one dose of study drug), the incidence and type of treatment- related adverse events were tabulated and summarized.
Kaplan- Meier techniques in SAS were used on the intent-to-treat population to assess time-to-event analyses such as PFS and survival and point probabilities every 6 months. Survival was calculated from the start of treatment to the date of death. The date of last contact was in place of a missing death date or for patients who were still alive.
Results
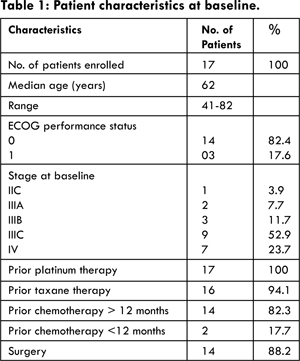
Patient Characteristics: Patient characteristics are listed in Table 1. Between January 2012 and March 2014, 17 patients enrolled onto the trial. Of particular note, the majority of patients had previously undergone surgical management (88.2%), and the majority had a diagnosis of recurrent disease more than 12 months since completion of initial chemotherapy. All 17 patients had previously received platinum, and 16 of 17 patients had previously received a taxane (either paclitaxel or docetaxel).
Treatment Outcomes: Patients were assessed with RECIST, CA-125, response rates are summarized in Tables 2 and 3. The objective response rate (CR and PR) was 58% (95% CI, 49.4% to 77.9%). When including patients with stable disease >6 months, a clinical benefit was demonstrated in 70.5% of eligible patients. The median time to a response was 1.3 months, and the median duration of the best response was 7.9 months. Responses, as measured by RECIST criteria or CA-125, are shown in Table 2. When assessing response using either RECIST criteria only (n=9) or CA-125 criteria only (n=8), a higher number of responses were noted in patients where the only measure of disease was RECIST (52% v/s 47%). To be characterized as a CR, patients had to meet CR criteria for both methodologies.
There were two patients who met CR criteria by CA-125 but had a PR by RECIST. At the conclusion of the study and after 18 months of follow-up, 12 patients (70.5%) were alive, with or without disease. There were seven deaths attributable to disease progression. Median overall survival had not been reached. The estimated median PFS was 8.5 months.
Toxicities for the treated patients are listed in Table 4. The major toxicity was hematologic (17.6% of patients’ experienced grade 3 or 4 neutropenia), with few grade 3 to 4 non-hematologic toxicities. Grade 3 neuropathy occurred in four patients (5.8%). There were no grade 4 neurologic toxicities. Generally, toxicities were mild to moderate and were manageable.
Discussion
In the treatment of recurrent epithelial ovarian cancer after initial platinum-based chemotherapy, sensitivity or resistance to platinum is an important factor in determining subsequent treatment. The period of time between the end of first-line treatment and subsequent relapse defines sensitivity to re-treatment with platinum [10,11]. Generally, the responses rates to platinum-based salvage treatment are 25%, 33%, and 60% at intervals of 6 to 12, 12 to 24, and more than 24 months. Re-treatment with a platinum regimen less than 6 months since prior platinum-based chemotherapy is associated with only a minor response to treatment. Several studies report re-treatment of platinum-sensitive patients with recurrent EOC with platinum-based regimens. Kavanagh et al. [12] reported ORR of 21%, with a median DoR of >7 months. Only those patients with platinum-free intervals >12 months who were initially responsive to a taxane responded to the reintroduction of carboplatin. Omura et al. [13] explored two doses of paclitaxel in 271 patients with recurrent EOC with ORR of 36% with 250 mg/m2 and 27% with 175 mg/m2. Median DoR was 13.1 months and 12.3 months, respectively. Borutaet et al. [14] administered salvage paclitaxel as a weekly low-dose infusion. In this group of 22 patients with advanced-stage disease, the ORR was 50% (27% CR and 23% PR rates). In a single-agent phase II study, 44 evaluable patients were treated with nab-paclitaxel 260 mg/m2 intravenously over 30 minutes every 21 days for 6 cycles or progression [15] with ORR of 64 and median PFS of 8.5 months. Severe adverse events were infrequent despite the dose, including 11% grade 4 neutropenia, 2% grade 3 fatigues and 13% grade 2–3 neuropathy. No hypersensitivity reactions were recorded. In the current study, the ORR was 59% (35% CR and 24% PR rates). Median time to response was 1.3 months, and median DoR was 7.9 months. The median OS has not yet been reached, and median PFS was 8.5 months. The response data in our trial compare favorably with those of other reports of patients with platinum-sensitive disease re-treated with platinum in combination with paclitaxel or gemcitabine or docetaxel. Myelosuppression in this study was also notably less frequent than that observed in studies of two-drug regimens. Although the current standard of care for patients with platinum-sensitive disease remains platinum-based combination therapy, the activity demonstrated in this study, the combination of nab-paclitaxel and platinum deserves further study. Grade 3 peripheral neuropathy was reported in approximately 5.9% of patients in this trial.
Nab-paclitaxel is highly active as a single agent in patients with platinum-sensitive recurrent ovarian cancer. The agent is well tolerated, with a favorable toxicity profile. The agent should be further studied in combination with platinum in first- and second-line treatment of patients with EOC.
References
- Seigel R, Naishadham D, Jemal A, Cancer statistics, 2013. CA cancer J Clin. 2013; 63:11-30.
- Ozols RF, Bundy BN, Greer BE, et al. Phase trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194-3200.
- Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al. Scottish Gynaecological Cancer Trials Group: Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682-1691.
- Papadimitriou CA, Fountzilas G, Aravantinos G, et al. Second-line chemotherapy with gemcitabine and carboplatin in paclitaxel-pretreated and platinum-sensitive ovarian cancer patients: A Hellenic Cooperative Oncology Group Study. Gynecol Oncol. 2004;92:152-159.
- Kose MF, Sufliarsky J, Beslija S, et al. A phase II study of gemcitabine plus carboplatin in platinumsensitive, recurrent ovarian carcinoma. Gynecol Oncol. 2005;96:374-380.
- Strauss HG, Henze A, Teichmann A, Karbe I, Baumgart A, et al. Phase II trial of docetaxel and carboplatin in recurrent platinum-sensitive ovarian, peritoneal and tubal cancer. Gynecol Oncol. 2007; 104:612-616.
- Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317-1324.
- Blum JL, Savin MA, Edelman G, Pippen, JE, Robert NJ, Geister, BV, et al. Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer. 2007;7:850-856.
- Rizvi NA, Riely GJ, Azzoli CG, Miller VA, Ng KK, Fiore J, et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non–small-cell lung cancer. J Clin Oncol. 2008;26:639-643.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2009-2106.
- Pfisterer J, Plante M, Vergote I, Andreas du Bois, Hal Hirte, Angel J. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: And intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006; 24:4699-4707.
- Kavanagh J, Tresukosol D, Edwards C, Freedman R, de Leon CG, Fishman A, et al. Carboplatin reinduction after taxane inpatients with platinum-refractory epithelial ovarian cancer. J Clin Oncol. 1995;13:1584-1588.
- Omura GA, Brady MF, Look KY, Averette HE, Delmore JE, Long HJ, et al. Phase III trial of paclitaxel at two dose levels, the higher dose accompanied by filgrastim at two dose levels in platinum-pretreated epithelial ovarian cancer: An intergroup study. J Clin Oncol. 2003;21:2843-2848.
- Boruta DM 2nd, Fowler WC Jr, Gehrig PA, et al. Weekly paclitaxel infusion as salvage therapy inovarian cancer. Cancer Invest. 2003;21:675-681.
- Teneriello MG, Tseng PC, Crozier M, Encarnacion C, Hancock K, Messing MJ, et al. Phase II evaluation of nanoparticle albumin-bound paclitaxel in platinum-sensitive patients with recurrent ovarian, peritoneal, or fallopian tube cancer. J Clin Oncol. 2009;27:1426-1431.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Singh RK, Pankaj S, Kota VR, Kumar SNab-Paclitaxel in Recurrent Ovarian Cancer: An Institution Based Retrospective Study.JCR 2015;5:94-99 |
|
Singh RK, Pankaj S, Kota VR, Kumar SNab-Paclitaxel in Recurrent Ovarian Cancer: An Institution Based Retrospective Study.JCR [serial online] 2015[cited 2025 Oct 18];5:94-99. Available from: http://www.casereports.in/articles/5/1/Nab-Paclitaxel-in-Recurrent-Ovarian-Cancer.html |

|
|
|
|
|