|
|
|
|
|
Response of Plasminogen Deficiency Associated Ligneous Conjunctivitis to Topical Fresh frozen Plasma with Heparin
|
|
|
symbicort asthma dose symbicort generic alternative read here
1Ophthalmology Department and 2Infection & Immunology Division, Ophthalmology Department, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia. |
|
|
|
|
|
Corresponding Author:
|
Dr. Samira Cut Putri
Email: putri.samira87@gmail.com
|
|
|
|
|
|
|
|
|
Received:
20-NOV-2014 |
Accepted:
27-FEB-2015 |
Published Online:
25-MAR-2015 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Introduction: Ligneous conjunctivitis is rare form of conjunctivitis characterized by woody fibrinous membranes. Plasminogen deficiency has been introduced as the most common cause and formation of membrane is the result of impaired wound healing process. Case Report: We present a 4-month-old male infant with chronic membranous conjunctivitis. Multiple scrapping of membranes were performed with no improvement. Fibrinous membranes were also seen on nasal cavity and upper gingiva. After his blood revealed plasminogen blood levels of 11.9% (normal: 75-135%), diagnosis of ligneous conjunctivitis due to plasminogen deficiency was obtained. Treatment was then changed to topical fresh frozen plasma and heparin. Dramatic resolution of membranes was observed after 15 months therapy combined with membranes scraping. Conclusion: Ligneous conjunctivitis is rare manifestation of plasminogen deficiency that occurs sporadically where local infection and multiple surgeries might act as trigger to induce fibrinous membranes. Topical heparin and FFP could treat the disease successfully.
|
|
|
|
|
|
Keywords :
|
Conjunctivitis, Plasminogen, Heparin, Nasal Cavity, Gingiva, Infant, Humans.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff341f060000008b03000001000500 6go6ckt5b5idvals|443 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Ligneous conjunctivitis (LC) is a rare manifestation of plasminogen deficiency that could occur sporadically in any mucous membranes of body
[1-3]. In most cases, LC is associated with infections of the upper respiratory tract, oral cavity, or ear in the early phase. Reduced levels of plasminogen functional assay and plasminogen antigen in plasminogen deficiency lead to development of fibrinous membranes with subsequent damage of affected tissue [4,5]. The severity of LC is related to the degree of plasminogen deficiency caused by homozygous or double heterozygous gene mutations transmitted as autosomal recessive trait [6-12].
Topical fresh frozen plasma (FFP) which contains plasminogen and heparin is shown to be effective in controlling membrane development [3,8]. Here we present a rare case of LC with delayed diagnosis and resulted in complications due to side effect of medication. However, after appropriate therapy with FFP (fresh frozen plasma) and heparin showed a good response and resolution of membranes.
Case Report
A 4-month-old male infant presented with bilateral chronic recurrent membranous conjunctivitis. He was the second child born to non-consanguineous parents with a history of first child dying of hydrocephalus. Scrapping of membranes and histopathology examination showed necrotic tissue rich-fibrin without granulation. However, the conjunctival membranes recurred immediately after scrapping. Multiple scrapping on both eyelids were performed every one to three months, with topical and oral antibiotic with steroids therapy for both eyes thereafter, but no significant improvement was seen. The membranes also changed to yellow-white and woody-like appearance that became more thick and dense after each scrapping [Fig.1].
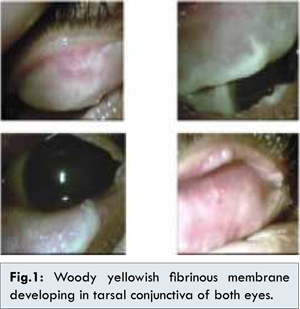
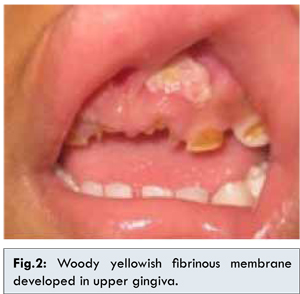
Two years later, the patients complained of photophobia and redness in right eye. Ophthalmological examination revealed corneal hazziness and shallow anterior chamber of right eye with increased intraocular pressure (IOP) in both eyes. Topical steroids were discontinued and IOP returned normal with 0.25% topical timolol and brinzolamide. Eventually, one year later the right eye developed thick posterior sub-capsular cataract. The patient also had history of recurrent green discharge and fibrinous membranes on the right nasal cavity. Histopathology examination of membranes in nasal cavity after sinus irrigation showed fibrotic stroma with inflammatory cells without sign of malignancy consistent with conjuctival membranes. At 5 years of age, fibrinous membranes also developed on upper gingival mucosa. Dental extraction was performed and histopathology examination of membranes in gingival showed necrotic tissue similar with membranes in conjunctiva and nasal cavity [Fig.2].
Laboratory examination revealed plasminogen blood concentration of 11.9% (normal: 75%-135%) which confirmed the definitive etiology of plasminogen deficiency. Treatment was then changed to topical heparin every three hours and fresh frozen plasma (FFP) every two hour. Fifteen months later, there was dramatic resolution of membranes in both eyes after scrapping along with topical FFP and heparin in both eyes. Patient also underwent phacoemulsification and intraocular lens implantation combined with right eye trabeculectomy. There has been no recurrence of membranes on tarsal conjunctiva in both eyes on two year follow-up with discontinuation of topical FFP and heparin [Fig.3]. This patient also consulted pediatric department for further examination regarding plasminogen deficiency that could affect all mucosal surface in the body.
Discussion
The diagnosis of LC from plasminogen deficiency is based on clinical sign, typical histological findings, laboratory examination of plasminogen level, and eventually on a positive family history [3,4,7-12]. In patient with plasminogen deficiency, wound-healing capability is markedly diminished and most pronounced in mucous membranes, such as conjunctiva which are rich in fibrin [4,14]. Histopathological examination of membranes shows a massive deposition of fibrin and amorphous hyalin-like eosinophilic material, accompanied by an inflammatory cellular infiltration. Fibrin deposition increases vascular permeability, influence the expression of inflammatory mediators and alter the migration and proliferation of various cell types [7,9]. Laboratory analysis should confirm this disorder with an abnormality of plasminogen activity. Activity and antigen testing is readily available in most clinical coagulation laboratories. The lower limit detection of plasminogen antigen in human plasma is 0.4 mg/dL, while the normal plasminogen blood level was 75-135%. The lower limit of plasminogen functional activity detection is 5% [14,15]. However, normal serum plasminogen antigen do not exclude LC [16]. A family history may potentially help support the diagnosis if other affected siblings or family members are available.
Genetic analysis in patients with LC demonstrates an autosomal recessive transmission and several plasminogen gene mutations. The human plasminogen gene is located on chromosome 6 [10]. On clinical grounds, identifying plasminogen gene mutations is useful to complete the diagnosis in these patients suffering from LC and in suspicious heterozygous carriers from the parents. It may also be used for prenatal diagnosis [4,10].
Watts et al. reported 3 cases of LC treated with topical plasminogen drops prepared from FFP. However, resolution of the membranes was insignificant [17]. Heidemann et al. were the first to treat a 7-year-old boy with topical plasmin, but then changed with topical plasminogen that showed better result [18]. The free plasmin in the circulation was immediately inhibited by plasmin inhibitors. Plasminogen, on the other hand, is not affected by these inhibitors and is incorporated into fibrin clot. Therefore, the use of plasminogen drops permits the conversion to plasmin as required to break down fibrin membranes. Despite these studies showed the effectiveness of plasminogen, however it is not commercially available, and it needs a complex procedure to be prepared [8,18].
Recent literature stated that heparin primarily acts by accelerating the activity of antithrombin and neutralizing factor Xa and thrombin, following exposure to triggers of ocular inflammation. Hence it prevents the accumulation of fibrin deposits commonly observed in LC. Azad et al. also used heparin because of its antifibrin action, and resulted in no recurrence of the lesion in 12 months of follow-up [19]. In other case, Gurlu et al. reported successful treatment of LC in infant with systemic and topical fresh frozen plasma with conjunctival membranes showing significant reduction in the first week of treatment [7]. Based on report above, our patient was treated with topical heparin and FFP. This treatment modality shortens the treatment period, reduces the probability of developing other systemic symptoms, and is a good alternative to plasminogen concentrates.
Surgery for pseudomembrane excision is often necessary, but it is affected by recurrences in almost all cases. This is probably due to fibrin membrane formation after surgical trauma that is not lysed in the presence of plasminogen deficiency [7,8,11,17]. Wats showed more stable results in three patients who underwent surgery with a combination of topical treatments with plasminogen drops [17]. Administering topical FFP and heparin before surgery was successful with a good control of symptoms. Therefore, surgical removal of pseudomembranes was considered mandatory [17].
The prognosis for this disease is variable based on the extent and site of the symptoms. Although many identified patients have lived into adulthood, a number of them have died from the effects of this disorder. The analysis of plasminogen activity is important regarding the death-risk complication from infection in children and to prevent misdiagnosis and wrong treatment. Complications of infection in respiratory tract could have resulted in respiratory failure. It is clear that the affected individual with plasminogen deficiency needs specific therapies that treat and prevent the ligneous lesion and its sequelae [3,4]. To prevent the systemic sequelae and evaluate the possible complications, this patient has been referred to pediatric department for further examination.
Unfortunately, two years after using different formulation of topical corticosteroids, there has been complication on the right eye that resulted in corticosteroid-induced glaucoma and posterior subcapsular cataract. To manage this complication, phacoemulsification with IOL implantation combined with trabeculectomy was choosen for this patient.
Conclusion
Local infection and multiple surgeries at site of the disease could act as the trigger to induce fibrinous membranes in ligneous conjunctivitis due to plasminogen deficiency. Topical heparin and FFP could manage and stabilize the recurrence of the membranes. Overall, we can learn a very valuable lesson from this case to keep possibility of ligneous conjunctivitis in children with refractory membranous conjunctivitis.
References
- Mehta R, Shapiro D. Plasminogen deficiency. Haemophilia. 2008;14:1261-1268.
- American Academy of Ophthalmology. Basic and Clinical Science Course: Section 8: External Disease and Cornea. San Fransisco: American Academy of Ophthalmology: 2009-2010.pp.213-214.
- Sowka JW, Gurwood AS, Kabat AG. Conjunctiva and sclera. In: The Handbook of ocular disease management. Supplement to review of optometry: 2011: pp. 12-20
- Rodriguez-Ares MT, Abdulkader I, Blanco A, Tourino-Peralba R, Ruiz-Ponte C, Vega A, et al. Ligneous conjunctivitis: a clinicopathological, immunohistochemical, and genetic study including?the treatment of two sisters with multiorgan involvement. Virchows Arch. 2007;451:815-821
- Siboni SM, Spreafico M, Menegatti M, Martinelli I, Pevyandi F. Molecular characterization of an Italian patient with plasminogen deficiency and ligneous conjunctivitis. Blood Coagul Fibrinolysis. 2007;18:81-84.
- Ciftci E, Ince E, Akar N, Dogru U, Tefs K, Schuster V. Ligneous conjunctivitis, hydrocephalus, hydrocele, and pulmonary involvement in a child with homozygous type I plasminogen deficiency. Eur J Pediatr. 2003;162:462-465.
- Gurlu VP, Demir M, Alimgil L, Erda S. Systemic and Topical Fresh-Frozen Plasma Treatment in a Newborn With Ligneous Conjunctivitis. Cornea. 2008;27:501-503.
- Caputo R, Pucci N, Mori F, Secci J, Novembre E, Frosin R. Long-term efficacy of surgical removal of pseudomembranes in a child with ligneous conjunctivitis treated with plasminogen eyedrops. Thromb Haemost. 2008;100:1196-1198.
- Putri DE, Edwar L, Susiyanti M. Acanthamoeba Keratitis in a Non-contact Lens Wearer: A Challenge in Diagnosis and Management. Journal of Case Reports. 2014;4(2):419-423.
- Sartori MT, Saggiorato G, Pellati D, Bello FD, Lombardi AM, Opocher G, et al. Difficulties in the Mutation Analysis of Plasminogen Gene: A Study in Two Patients with Ligneous Conjunctivitis. Clin Appl Thrombosis/Hemostasis. 2006; 12(1):77-84.
- Kraft J, Lieb W, Zeitler P, Schuster V. Ligneous conjunctivitis in a girl with severe type I plasminogen deficiency. Graefe’s Arch Clin Exp Ophthalmol. 2000;238:797-800.
- Schuster V, Hugle B, Tefs K. Plasminogen deficiency. Journal of Thrombosis and Haemostasis. 2007;5:2315-2322.
- Kansky JC. Conjunctiva. In: Clinical ophthalmology A systematic approach.Fifth edition. Edinburgh; Butterworth Heineman: 2003. pp.65-70.
- Chen S, Wishart M, Hiscott P. Ligneous conjunctivitis: A local manifestation of a systemic disorder? J AAPOS 2000;4:313-315.
- Schuster V, Seidenspinner S, Zeitler P, Escher C, Pleyer U, Bernauer W, et al. Compound-Heterozygous Mutations in the Plasminogen Gene Predispose to the Development of Ligneous Conjunctivitis. Blood. 1999;93:3457-3466.
- Fuentez-Paez G, Herreras JM, Mendez MDC, Saorni MA. Ligneous conjunctivitis in a patient with Crohn’s disease. Clinical Ophthalmology. 2008:2(1):203-206.
- Watts P, Suresh P, Mezer E, Ells A, Albisetti M, Bajzar L, et al. Effective Treatment of Ligneous Conjunctivitis With Topical Plasminogen. Am J Ophthalmol. 2002;133:451-455.
- Heidemann DG, Williams GA, Hartzer M, Ohanian A, Citron ME. Treatment of Ligneous Conjunctivitis with Topical Plasmin and Topical Plasminogen. Cornea. 2003;22:760-762.
- Azad N, Zafar S, Khan A. Successful treatment of ligneous conjunctivitis with topical cyclosporine and heparin. J AAPOS 2009;13:519-520.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Putri SC, La Distia NR, Made SResponse of Plasminogen Deficiency Associated Ligneous Conjunctivitis to Topical Fresh frozen Plasma with Heparin.JCR 2015;5:132-136 |
|
Putri SC, La Distia NR, Made SResponse of Plasminogen Deficiency Associated Ligneous Conjunctivitis to Topical Fresh frozen Plasma with Heparin.JCR [serial online] 2015[cited 2025 Dec 28];5:132-136. Available from: http://www.casereports.in/articles/5/1/Response-of-Plasminogen-Deficiency-Associated-Ligneous-Conjunctivitis-to-Topical-Fresh-frozen-Plasma-with-Heparin.html |

|
|
|
|
|