Introduction
Tumoral calcinosis (TC) is a rare clinical and histopathologic syndrome characterized by calcium salt deposition in different peri-articular soft tissue regions [
1]. It mainly manifests in childhood or adolescence as painless, firm, tumor-like masses around the joints that may lead to joint function limitations [
1-
3]. Sometimes ulceration of the overlying skin can occur with superadded secondary infection [4].
Growth of TC lesions is mostly slow and progressive in nature over several years [
3]. Hip and shoulder joints are most commonly affected; however, spinal [
5], temporo-mandibular joint [
6], metacarpals/metatarsals [
7] and popliteal space [
8] involvement has also been reported. In this article, we report two cases of recurrent primary TC. This is followed by a comprehensive review of literature regarding the current understanding of the pathogenesis and the available diagnostic and treatment modalities.
Case Reports
Case 1
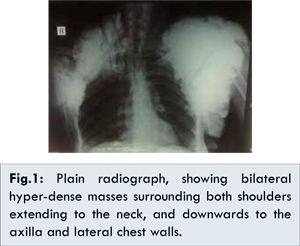
A 35-year-old female patient presented with a slowly progressive swelling around the left shoulder since 5 years. He had a history of partial excision of a mass in the left axilla one and a half year back which was followed by rapid recurrence after one month. Another swelling appeared around the right shoulder since one year with the same features (painless, hard and fixed to the joint with irregular outline). Both masses caused limited mobility of the shoulder joints and the right mass resulted in skin necrosis. The patient gave no history of medical illness or family history of similar conditions. Plain radiograph showed bilateral hyperdense masses surrounding both shoulders extending to the neck, and downwards to the axilla and lateral chest wall [Fig.1].
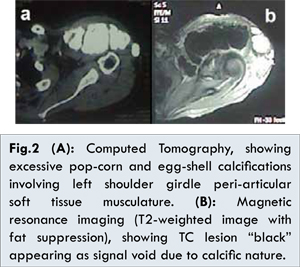
Computed tomography (CT) of the shoulders showed bilateral excessive popcorn and egg shell calcifications involving the peri-articular shoulder girdle musculature. On the right side, they were seen compressing the Scalene and Serratus muscles, while on the left side they were compressing the Trapezius, Supraspinatus and Deltoid muscles, respecting the margins of the affected muscles and not compromising the interlacing fat planes replacing all affected muscle parenchyma [Fig.2a]. Magnetic resonance imaging (MRI) confirmed the CT findings [Fig.2b]. The biochemical profile of the patient showed normal renal function tests, normal parathormone level (46.00 pg/mL, normal range: 8.7-79.6 pg/mL), normal total serum calcium level (8.62 mg/dL, normal range: 8.4 -10.2 mg/dL) and a high serum phosphorus level (7.96 mg/dL, normal range: 2.50-4.50 mg/dL).
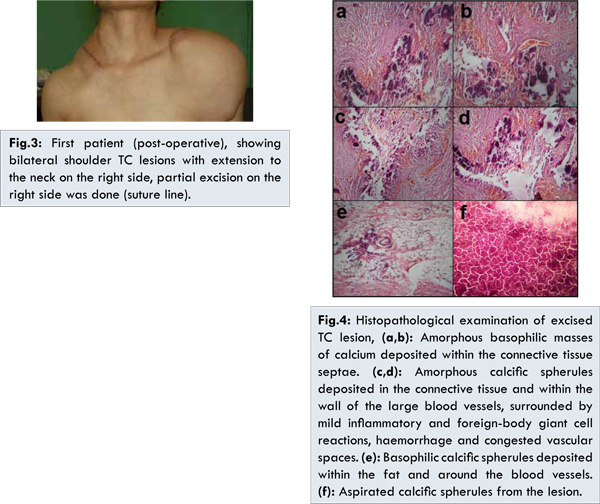
Partial excision of the right shoulder mass for debulking and pain relief was done. Intra-operatively the mass on the right side was approached through an elliptical supraclavicular incision. It was dissected from the surrounding soft tissue till its extension beyond the clavicle where complete excision proved to be impossible without neural injury. Thus, shaving at the level of the clavicle was performed [Fig.3]. Histopathological examination of the excised mass confirmed the diagnosis of TC [Fig.4].
One month postoperatively recurrence of the excised part occurred and the patient was prescribed medical treatment in the form of phosphorus binding antacids and dietary phosphorus restriction. Despite on medical treatment; three months postoperatively, the patient showed ulceration of the overlying skin with bilateral sinus formation showing calcific discharge and superadded infection occurred on the right side. Frequent dressing and antimicrobials were started to control the infection, which finally resolved. The lesions showed no improvement on medical treatment during a 5-month postoperative follow up. Further follow-up was not achieved due to the patient’s non-compliance.
Case 2
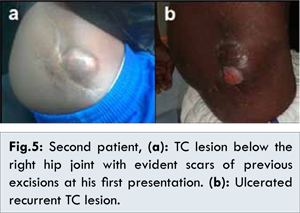
A 30-year-old male patient presented to surgery department in February 2013, with a recurrent swelling just below the right hip joint after two previous surgical excisions (in 2004 and 2006) [Fig.5a], that proved to be TC by histopathologic examination. The swelling measured approximately 5x3 cm in size, was hard in consistency and immobile. The patient was a known epileptic controlled by medical treatment with phenytoin and valproic acid. Plain radiograph of the hip joint showed a hyper-dense calcified mass overlying the greater trochanter, while CT scan showed corrugated dense ossified mass seen in the subcutaneous tissue overlying the right greater trochanter, measuring about 3x2x4.8 cm in dimensions with no associated extension into deeper structures. The biochemical profile showed normal levels of total serum calcium (8.9 mg/dL, normal range: 8.4-10.2 mg/dL), phosphorus (3.2 mg/dL, normal range: 2.50-4.50 mg/dL) and parathormone (41 pg/mL, normal range: 8.7-79.6 pg/mL). Complete surgical excision of the mass, with the overlying scarred adherent skin was performed through an elliptical incision. A one centimetre margin of grossly normal tissue was excised with the lesion. The diagnosis of TC was confirmed by histopathological examination. In September, 2013 the patient returned with a recurrence at the same site with ulceration of the overlying skin [Fig.5b]. This time the lesion was smaller and fixed to underlying muscles. Frequent dressings were done for the ulcerated part, and complete healing eventually occurred. The patient was discharged on phosphate binders and a phosphate-restricted diet, till present time. Follow-up showed no regression in size of the lesion.
In 1996, Smack et al. [
9], reviewed the literature and described three distinct types of TC namely; primary normo-phosphatemic TC with no familial base, primary hyper-phosphatemic TC with positive familial correlation, and the secondary type associated with an underlying calcifying process (most commonly; chronic renal failure). The familial base for the primary hyper-phosphatemic type is attributed to mutations in GALNT3, KLOTHO or FGF23 genes resulting in malfunction of FGF23 and reduced urinary phosphate excretion [
10]. A sequence of local events was suggested to underlie the characteristic TC lesions. Minor or persistent trauma to the affected site results in a foamy histiolytic reaction, which leads to neo-bursae formation. Mature bone formation is hindered by collagenolysis initiated by the products of disintegrated histiocytes, leading to the formation of the characteristic TC lesions (active stage). Finally, calcified debris fills the loculi ending in bone formation with arrest of bursae formation. Decline in collagenolysis results in fibrosis that surrounds the TC lesions (quiescent stage) [
11]. While hyper-phosphatemia (the drive behind the calcification process) is evident in the hyper-phosphatemic type, transient hyper-phosphatemia is accused in the normo-phosphatemic type resulting from local tissue trauma or exogenous phosphate-saline laxative intake [
11,
12]. Huge bilateral cases (such as the first patient) have been rarely described in the literature [
13,
14].
Diagnosis of TC is a clinico-radiological one in the first place. Although biopsy is frequently required to distinguish TC from its mimics, open tissue biopsy can end in fistula track formation and infection [
14]. Plain radiographs show typical juxta-glandular multi-cystic calcifications. Computed tomography delineates the extent and relations of the lesions guiding for surgical planning, It usually shows cystic loculi with fluid-fluid levels caused by calcium layering “the sedimentation sign’ and no erosion or osseous destruction, another hallmark of TC [
15,
16]. Magnetic resonance imaging (MRI) can be done in difficult cases showing inhomogeneous high signal intensity on T2-weighted sequences with two patterns commonly observed; diffuse lower-signal-intensity pattern, or nodular pattern with alternating areas of high signal intensity and signal void. On T1-weighted sequences, the lesions appear inhomogeneous with low-signal intensity [
17]. Scintigraphy reflects the activity of the lesions and can detect early lesions and bone marrow affection [
15,
17]. Correlation with the history and biochemical profile is mandatory before confirming the diagnosis. Typically serum calcium level, serum phosphorus level, parathormone level, renal function tests and 1,25-dihydroxy–vitamin-D level should be done in every patient [
15]. A negative antinuclear, anti-Smith, anti-centromere and anti-scleroderma antibodies profile would augment the diagnosis through exclusion of an underlying connective tissue disease [
3,
15]. No diagnostic difficulty was encountered in the presented cases because the clinical and radiological features were typical of TC and both were recurrent with documented histopathological diagnosis of previous excisions.
Treatment of the primary type has been classically described to be surgical with complete excision being the ultimate goal. However, surgery is associated with high recurrence, failure of complete excision, persistence of etiology, high complication rate and secondary infection [
18,
19]. The second line of treatment is a medical one in the form of phosphate binders, phosphate-restricted diet [
7,
18] and acetazolamide [
20]. Alternative modalities including the administration of steroids, diphosphonates, calcitonin or radiation therapy have not proven to be effective [
21,
22].
A wider surgical resection margin is postulated to be able to decrease the possibility of recurrence due to hyper-vascular region beyond the periphery of the calcified TC lesion [
23]. This theory needs to be further augmented, as excision of the lesion in the second patient with a one centimetre safety margin didn’t prevent recurrence. Restricting surgical excision to cases with disabling symptoms seems reasonable. A stage-oriented approach taking in consideration the underlying TC pathogenesis including using medical treatment in the active stage and surgical treatment in the quiescent stage can be considered. Also, a combination treatment of surgical and medical treatment is advocated by some authors in resistant cases [
24].
Conclusions
Despite being a benign pathology, TC can be a highly distressing and disabling condition to the patient in the advanced cases. Total surgical excision was not feasible in the first patient due to intimate neurovascular relations, treatment with phosphate binders also failed. Complete excision with a safety margin didn’t prevent recurrence in the second patient. These findings warrant seeking other alternatives and further investigating the possible causes of local recurrences.
Authors Contributions
FI and SM: collected the data and wrote the manuscript. NA and HY: reviewed the literature and managed the patients. All authors read and approved the final manuscript.
References
- McClatchie S, Bremner AD. Tumoral calcinosis--an unrecognized disease. Br Med J. 1969;1(5637):153-155.
- Pakasa NM, Kalengayi RM. Tumoral calcinosis: a clinicopathological study of 111 cases with emphasis on the earliest changes. Histopathol. 1997;31(1):18-24.
- Fathi I, Sakr M. Review of tumoral calcinosis: a rare clinico-pathological entity. World J Clin Cases. 2014;2:409-414.
- Geirnaerdt MJ, Kroon HM, van der Heul RO, Herfkens HF. Tumoral calcinosis. Skeletal Radiol. 1995;24(2):148-151.
- Durant DM, Riley LH 3rd, Burger PC, McCarthy EF. Tumoral calcinosis of the spine: a study of 21 cases. Spine. 2001;26(15):1673-1679.
- Gal G, Metzker A, Garlick J, Gold Y, Calderon S. Head and neck manifestations of tumoral calcinosis. Oral Surg Oral Med O. 1994;77(2):158-166.
- Mozaffarian G, Lafferty FW, Pearson OH. Treatment of tumoral calcinosis with phosphorus deprivation. Ann Int Med. 1972;77(5):741-745.
- Giardina F, Sudanese A, Bertoni F, Guerra E, Paderni S. Tumoral calcinosis of the popliteal space. Orthop. 2004;27(10):1104-1107.
- Smack D, Norton SA, Fitzpatrick JE. Proposal for a pathogenesis-based classification of tumoral calcinosis. Int J Dermatol. 1996;35(4):265-271.
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, et al. Mutations in GALNT3 encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579-581.
- Slavin RE, Wen J, Barmada A. Tumoral calcinosis: a pathogenetic overview: a histological and ultrastructural study with a report of two new cases, one in infancy. Int J Surg Pathol. 2012;20(5):462-473.
- Markowitz GS, Perazella MA. Acute phosphate nephropathy. Kidney Int. 2009;76(10):1027-1034.
- Rifi M, Kharmaz M, Bouchida A, Jahid A, El Bardouni A, Lamrani M, et al. Calcinose tumorale idiopathique bilaterale de la region Trochanterienne : a propos d’un cas et revue de la litterature. Rev Maroc Chir Orthop Traumato. 2008;34:48-50.
- Farzan M, Farhoud AR. Tumoral calcinosis: what is the treatment? Report of two cases of different types and review of the literature. Am J Orthop. 2011;40(9):E170-6.
- Olsen KM, Chew FS. Tumoral calcinosis: pearls, polemics, and alternative possibilities. Radiographics. 2006;26(3):871-885.
- Hug I, Guncaga J. Tumoral calcinosis with sedimentation sign. Br J Radiol. 1974;47:734-736.
- Martinez S, Vogler JB, Harrelson JM, Lyles KW. Imaging of tumoral calcinosis: new observations. Radiol. 1990;174:215-222.
- Kirk TS, Simon MA. Tumoral calcinosis. Report of a case with successful medical management. The J Bone Joint Surg Am. 1981;63(7):1167-1169.
- King JJ, Brennan KB, Crawford EA, Fox EJ, Ogilvie CM. Surgical complications associated with extensive tumoral calcinosis. Am J Orthop. 2011;40(5):247-252.
- Yamaguchi T, Sugimoto T, Imai Y, Fukase M, Fujita T, Chihara K. Successful treatment of hyperphosphatemic tumoral calcinosis with long-term acetazolamide. Bone. 1995;16(4 Suppl):247S-50S.
- Inclan A, Leon PP, Camejo M. Tumoral calcinosis. J Am Med Ass. 1943;121(7):490-495.
- Kallmeyer JC, Seimon LP, MacSearraigh ET. The effect of thyrocalcitonin therapy and phosphate deprivation on tumoral calcinosis. S Afr Med J. 1978;54(23):963-966.
- Neeman Z, Wood BJ. Angiographic findings in tumoral calcinosis. Clin Imag. 2003;27(3):184-186.
- Hamada J, Tamai K, Ono W, Saotome K. Uremic tumoral calcinosis in hemodialysis patients: clinicopathological findings and identification of calcific deposits. J Rheumatol. 2006;33(1):119-126.