Introduction
Holoprosencephaly (HPE) results from failure of the prosencephalon to differentiate into the cerebral hemispheres and lateral ventricles between the fourth and eighth week of gestation [
1,
2]. HPE has an incidence rate of 1:250 in utero. However, the live birth rate is 1:16,000 [
1,
2]. This discrepancy is due to a high number of intrauterine deaths. Holoprosencephaly is classified into 4 types depending on the degree of involvement of the forebrain and include: alobar, semilobar, lobar and a middle interhemispheric fusion variant. Holoprosencephalic patients usually have various form of developmental delay depending upon severity and types. We describe a case of a term newborn diagnosed with lobar type of holoprosencephaly and briefly discuss the cranial and MRI imaging features.
Case Report
A term, 2600 grams baby was born at 37 weeks of gestational age to a 30 year old primigravida by normal vaginal delivery in peripheral centre. The APGAR score was normal at the time of birth. The head circumference and vital signs were within normal limits. The maternal history was unremarkable for any prenatal infections, trauma, drug abuse or any other chronic disease. No significant obstetric or family history was elicited. Baby subsequently developed sepsis and was transferred to our centre for the further management. On clinical examination body temperature of baby was 1010F, HR: 90 beats/min and RR: 20/min. Laboratory examination showed leucocytosis.
Prenatal cranial ultrasound of the baby showed bilateral symmetrical dilatation of posterior horns of lateral ventricles with poorly visualized frontal horns. Midline septum pellucidum was not visible with well-formed interhemispheric fissure. Thalami were separated with no obvious fusion of basal ganglia or thalamus. Sagittal imaging of cranial sonography revealed absence of normal corpus callosum with dysplastic non-uniform posterior callosal body and splenium. Rests of the brain parenchyma revealed no remarkable abnormality [Fig.1].
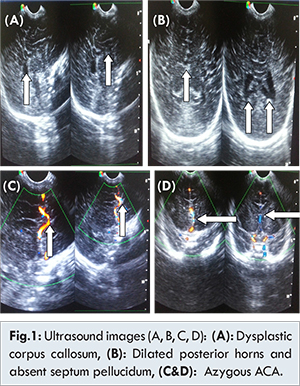
Colour Doppler imaging revealed single azygous anterior cerebral artery in the anterior interhemispheric space with normal circle of Willis. For further evaluation MR brain was performed. MRI brain confirmed all the sonographic imaging features with absence of septum pellucidum, well formed interhemispheric fissure, unfused basal ganglia & thalami, dilated posterior and dysplastic anterior horns of lateral ventricles and dysplastic corpus callosum. Azygous anterior cerebral artery was well visualized on MRI with single vascular flow void in anterior interhemispheric space of brain. Additional fused cingulated gyrus was noted on MR imaging of brain [Fig.2].
Discussion
Holoprosencephaly (HP) results from failure of the prosencephalon to differentiate into the cerebral hemispheres and lateral ventricles between the fourth and eighth week of gestation [
1-
3]. A spectrum of defects of malformations of the brain and face exists, which range from complete to partial failure of cleavage of the prosencephalon. Depending on the degree of disordered prosencephalic cleavage, HP is usually categorized as alobar, semilobar or lobar [
4].
Alobar type, there is no cleavage of the forebrain into right and left hemispheres, into the diencephalon and telencephalon and into the olfactory and optic bulbs. It results in a single, amorphous ventricle, fused thalami and absence of the corpus callosum, falx cerebri, optic tracts and olfactory bulbs [
3,
4]. Semilobar HP, in which the brain’s hemispheres have a slight tendency to separate, is an intermediate form of the disease. It shares many of these same features but demonstrates partial segmentation of the ventricles and incomplete fusion of the thalami [
3,
4]. Lobar HP, in which there is considerable evidence of separate brain hemispheres, is the least severe form. The etiology of HP is heterogeneous. Most cases occur sporadically and have a normal karyotype. However, the disorder can be associated with a variety of chromosomal abnormalities such as trisomy 13, ring chromosomes and deletions [
1,
4-
6]. The disorder can be familial, the mode of inheritance having been reported to be both autosomal recessive and autosomal dominant [
1,
5,
7,
8]. Alobar and semilobar have poor prognosis while lobar has variable mental impairment [
9-
11]. Our case was lobar type of HP diagnosed on cranial ultrasound and confirmed by MRI examination.
Sonographic features in lobar holoprosencephaly are absence of mono-ventricular cavity and fusion of thalami with considerable evidence of separated brain hemispheres. The two hemispheres are separated anteriorly and posteriorly with a certain degree of fusion of structures such as the lateral ventricles and cingulate gyrus and absence of the cavum septum pellucidum [
12]. The interhemispheric fissure is well formed and unlike alobar and semilobar HP, the lateral ventricles are only fused anteriorly. These findings are also associated with agenesis of the corpus callosum and septo-optic dysplasia [
13]. In our case sonographic features were similar with bilateral symmetrical dilatation of posterior horns of lateral ventricles with poorly visualized & partially fused frontal horns. Midline septum pellucidum was not visible with well-formed interhemispheric fissure. Thalami were separated with no obvious fusion of basal ganglia or thalamus. Sagittal imaging of cranial sonography revealed absence of normal corpus callosum with dysplastic non uniform posterior callosal body and splenium. Diagnosis of lobar HP is difficult because it relies mainly on the absence of the cavum septum pellucidum, together with variable enlargement of the lateral ventricles. MR imaging can accurately depict the mild lobar form of holoproscencephaly [
14]. Common MR imaging features in case of lobar holoproscencephaly are absence of septum pellucidum, well formed interhemispheric fissure, unfused basal ganglia & thalami, fused anterior horns of lateral ventricles and dysplastic corpus callosum [
14]. Azygous ACA can be found on MRI [
15]. Additional fused cingulated gyrus is noted on MR imaging of brain. Similar MRI features of lobar holoproscencephaly were present in our case including absence of septum pellucidum, well formed interhemispheric fissure, unfused thalami, fused cingulated gyrus, dilated posterior and dysplastic anterior horns of lateral ventricles and dysplastic corpus callosum. Azygous ACA is well documented on MRI in our case.
Pilu G et al. identified lobar holoprosencephaly in 12 fetuses between 21 and 35 weeks’ gestation on prenatal sonography [
16]. Early diagnosis of this condition prenatally helps in counselling of parents and future management of patient.
Conclusion
Holoprosencephaly (HP) is a congenital anomaly characterized by lack of cleavage of the prosencephalon. Although relatively rare, it is the most common anomaly that involves both the brain and the face. Diagnosis of this anomaly using ultrasonography, particularly of the less severe form of lobar type, is often challenging. We diagnosed the case of lobar holoproscencephaly on cranial sonography and performed MRI to confirm sonographic features. Early diagnosis of this entity is important for proper counselling of parents and future management of patient.
References
- DeMyer W. Holoprosencephaly (cyclopia-arhinencephaly). In: Vinken PJ, Bruyn GW, Klawans HL. eds. Handbook of clinical neurology. Amsterdam; Elesevier North Holland Biomedical Press. 1987;225-244.
- Babcock DS. Sonography of congenital malformations of the brain. Neuroradiology. 1986;28:428.
- Filly RA, Chinn DH, Callen PW. Alobar holoprosencephaly: ultrasonographic prenatal diagnosis. Radiology. 1984;151:455-459.
- Chervenak FA, Isaacson G, Hobbins JC, Chitkara U. Diagnosis and management of fetal holoprosencephaly. Obstet Gynecol. 1985;66:322-326.
- Cohen MM. An update on the holoprosencephalic disorders. J Pediatr. 1982;101:865-869.
- Nyberg DA, Mack LA, Bronstein A, Hirsch J, Pagon RA. Holoprosencephaly: prenatal sonographic diagnosis. Am J Roentol. 1987;149:1051-1058.
- Roach E, Demyer W, Conneally PM, Palmer C, Merritt AD. Holoprosencephaly: birth data, genetic and demographic analysis of 30 families. Birth defects. 1975;11:294-313.
- Dallaire L, Clarke Fraser F, Wiggleswarth FW. Familial holoprosencephaly. Birth defects. Original article, series VII, 1971;7:136-142.
- DeMeyer W. Classification of cerebral malformations. Birth defects: original article series; 1971;7:78-93.
- Pilu G, Reece EA, Romero R, Bovicelli L, Hobbins JC. Prenatal diagnosis of craniofacial malformations with ultrasonography. Am J Obstet Gynecol. 1986:155:45-50.
- Cayea PD, Balcar I, Alberti O Jr, Jones TB. Prenatal diagnosis of semilobar holoprosencephaly. Am J Roentol. 1984;142:401-402.
- Fowlie A, Constantine G. Holoprosencephaly, the central nervous system. In: Dewbury K, Meire H, Cosgrove D (eds.). Ultrasound in obstetrics and gynaecology. Churchill Livingstone 1993; 292-294.
- Byrd SE, Harwood-Nash DC, Fitz CR, Rogovitz DM. Computed tomography evaluation of holoprosencephaly in infants and children. J Comput Assit Tomogr. 1977;1:456-463.
- Wong Alex MC, Bilaniuk LT, Ng KK, Chang YL, Chao AS, Wai YY. Lobar holoprosencephaly: Prenatal diagnosis with post natal MR correlation. Prenat Diagn. 2005;25:296-299.
- Hahn JS, Barnes PD, Clegg NJ, Stashinko EE. Septopreoptic Holoprosencephaly: A Mild Subtype Associated with Midline Craniofacial Anomalies. Am J Neuroradiol. 2010;31:1596-1601.
- Pilu G, Sandri F, Perolo A, Giangaspero F, Cocchi G, Salvioli GP, Bovicelli L. Prenatal diagnosis of lobar holoprosencephaly. Ultrasound Obstet Gynecol. 1992;2(2):88-94.