|
|
|
|
|
Malignant Pleural Effusion of Multiple Myeloma: Prognostic Factors and Outcome.
|
|
|
Ajaz Ahmad Malik, Alshammari Sereihan, Javeed Iqbal
Department of Medical Oncology, King Abdul Aziz Specialist Hospital, Sakaka KSA. |
|
|
|
|
|
Corresponding Author:
|
Dr. Ajaz Ahmad Malik
Email: ejreen@gmail.com
|
|
|
|
|
|
|
|
|
Received:
26-OCT-2015 |
Accepted:
28-MAR-2016 |
Published Online:
10-JUN-2016 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Multiple myeloma (MM) is a plasma cell disorder marked by clonal proliferation of plasma cells. Multiple myeloma is one of the most common hematological malignancies seen worldwide. Pleural effusions are very rarely associated with multiple myeloma and most often signify a concurrent disease process and poor prognosis. |
|
|
|
|
|
Keywords :
|
Lymph Nodes, Multiple Myeloma, Plasma Cells, Plasmacytoma, Pleural Effusion.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff883b0d0000007503000001000400 6go6ckt5b5idvals|617 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Malignant pleural effusions in multiple myeloma (MM) are extremely rare, occurring in less than 1% of cases of multiple myeloma [1]. Pleural effusion in MM normally indicates a poor prognosis for myeloma and a low possibility of survival. Pleural effusion is seen in only few patients with myeloma. Myelomatous pleural effusions may arise from either extension of plasmacytomas of the chest wall, invasion from adjacent skeletal lesions, direct pleural involvement by myeloma (myelamatous pleural effusion, MPE) or following lymphatic obstruction secondary to lymph node infiltration [1,2]. Myelomatous pleural effusions are rare and only about 80 cases have been reported in English medical literature. In this paper, we report a Saudi patient with relapsed myeloma who developed myelomatous pleural effusion.
Case Report
A 59 year old known hypothyroid male on 125 µg L-thyroxine presented with shortness of breath and constitutional symptoms as generalized fatigue, 20 kg weight loss over 10 months. He was initially evaluated in Jordan 10 months back for bilateral pleural effusion which was drained with no available report mentioning the cause. He also had bilateral upper arm pathological fracture and underwent internal fixation in Jordan.
His baseline investigations showed Hb 9.8 (13-17 g/dL), platelets 184 (150-400 x 109/L), WBC 3.8 (4-10 x 109/L), urea 11 (6-25 mg/dL), creatinine 1.1 (0.8-1.3 mg/dL), LDH 767 (50-150 U/L) and positive Bence-jones protein. Viral screening was negative, for further work up serum protein electrophoresis was done. Peripheral blood smear showed pancytopenia with no rouleax formation or plasma cells seen. His chest X-ray was suggestive of bilateral pleural effusion with ill-defined ground glass opacity and basal atelectasis with multiple lytic lesions. Multiple compression fractures were noted through dorsal vertebrae with degenerative disc disease at level C4-C6.
MRI spine was not done due to implanted metals. CT scan lumbo-sacral spine was done which showed extensive intraspinal soft tissue mass extending from the level of C5 down to T12 displacing the cord anteriorly and causing severe spinal cord stenosis. There was also involvement of bilateral neural foramina along the entire length of mass interval with increased pleural effusion and paraspinal mass in the lower chest. Bone marrow aspiration revealed diffuse infiltration of plasma cell myeloma with immunohistochemistry testing positive for CD38, CD138 and lambda; and negative for CD20 and CD 56 and kappa.
He received one session concentrated dose of spinal radiotherapy and subsequent one cycle of chemotherapy based on (VCD) bortizomib, cyclophosphamide and dexamethasone. The initial plan from the treating team was to complete the 6 to 8 cycles in order to achieve complete remission or at least very good partial remission and then to arrange for autologous hematopoietic stem cell transplant. However 3 months after his initial treatment, he was admitted under oncology in ICU with Type 2 respiratory failure. Chest surgeon and chest physician were involved as he was found to have bilateral massive pleural effusion. Left effusion was drained which was hemorrhagic and was sent for cytology and histopathology examination (HPE). Right sided chest tube was inserted which again revealed hemorrhagic pleural fluid. His breathing difficulty improved after chest tube insertion, pleural fluid report turned out to be malignant effusion with the presence of plasmoid cells [Fig.1,2]. He completed his 3 sessions of chemotherapy after that he was reevaluated. Bone marrow histology showed infiltration with plasma cells. He had an IgG kappa paraprotein of 76.3 g/L. it was found that he is not responding to the chemotherapy protocol. His pleural effusion kept on re-accumulating. He was started on 2nd regimen of chemotherapy based on bortizomib, thalidomide and cyclophosphamide. However even after 2 cycle of chemotherapy he did not improve clinically and died in November 2015.
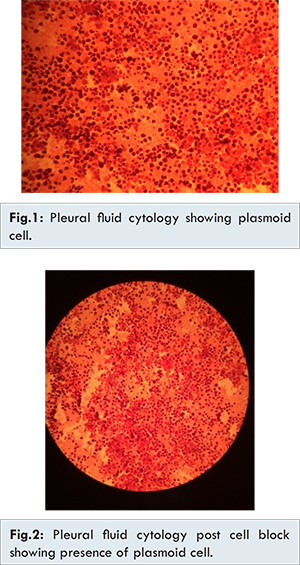
The development of pleural effusions in multiple myeloma is unusual. Kintzer et al. reported the incidence of pleural effusions in patients with multiple myeloma as 6% [2]. Furthermore, pleural effusions presenting in multiple myeloma are seldom a direct consequence of the myeloma itself, more often the result of a concurrent disease process or coexisting illness. Indeed, malignant myelomatous pleural effusions are rarely observed, occurring in less than 1% of cases [2]. Myelomatous pleural effusions may arise from either extension of plasmacytomas of the chest wall, invasion from adjacent skeletal lesions, direct pleural involvement by myeloma (pleural plasmacytoma) or following lymphatic obstruction secondary to lymph node infiltration [3-5]. The presence of an IgA paraprotein is most commonly associated with myelomatous pleural effusions, (in upto 80% of cases in some studies) [3,5]. The development of myelomatous pleural effusions is frequently a late complication of the disease and is associated with poor prognosis, with previous studies reporting median survival of less than 4 months [6,7]. It is interesting to note that in our case, histological analysis demonstrated presence of plasmoid cells. This may be an important contributory factor underlying the development of myeloma with malignant pleural effusions and may explain the aggressive nature of myelomatous disease that presents in this way. The development of extramedullary plasmacytomas (EMPs) in the context of pre-existing multiple myeloma occurs infrequently with only 5% of patients with EMPs having coexisting multiple myeloma [8,9].
In our case patient presented to us with respiratory failure and he had neglected treatment. He had undergone internal fixation of both the humerus bones due to pathological fracture in Jordan and had neglected further evaluation as to see the cause of his fractures. VCD remains the one of the first line treatments for transplant eligible candidates. However in our case patient did not respond to either of our therapy. Our experience suggests that pleural involvement with myeloma cells is associated with an aggressive course which is poorly responsive to first or second-line therapies used in conventional myeloma treatment.
Conclusion
The case discussed here reinforces that we should not become complacent when investigating pleural effusions in patient with a history of multiple myeloma. Malignant pleural effusion in patients with MM is often associated with poor prognosis despite aggressive local and systemic treatment.
Acknowledgements
We thank Mr. Ahmad Khalaf Muhammad Al Sharaan, Senior Lab Specialist, HPE for providing us the histopathology pictures.
References
- Kintzer JS Jr, Rosenow EC 3rd, Kyle RA. Thoracic and pulmonary abnormalities in multiple myeloma. A review of 958 cases. Arch Intern Med. 1978;138(5):727-730.
- Agrawal R, Kumar P. Multiple myeloma presenting as bilateral pleural effusion. Journal of Case Reports. 2012;2(2):48-50.
- Rodriguez J, Pereira A, Martinez JC, Conde J, Pujol E. Pleural effusion in multiple myeloma. Chest. 1994;105:622-624.
- Gogia A, Agarwal PK, Jain S, Jain K. Myelomatous pleural effusion. J Assoc Physicians India. 2005;53:734-736.
- Yokoyama T, Tanaka A, Kato S, Aizawa H. Multiple myeloma presenting initially with pleural effusion and a Unique Paraspinal Tumor in Thorax. Intern Med. 2008;47:1917-1920.
- Kamble R, Wilson CS, Fassas A, Desikan R, Siegel DS, Tricot G, et al. Malignant pleural effusions of myeloma: prognostic factors and Outcome. Leukaemia and Lymphoma. 2005;46:1137-1142.
- Dhingra KK, Singhal N, Nigam S, Jain S. Unsuspected multiple myeloma presenting as Bilateral pleural Effusion-A Cytological diagnosis. Cyto Journal. 2007;4:17-20.
- Moulopoulos LA, Granfield CA, Dimopoulos MA, Kim EE, Alexanian R, Libshitz HI. Extraosseous multiple myeloma: Imaging Features. AJR. 1993;161:1083-1087.
- Cabrera A, Klein JS. Bilateral pleural masses and shortness of breath associated with multiple myeloma. Chest. 1997;111:1750-1753.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Ahmad Malik A, Sereihan A, Iqbal JMalignant Pleural Effusion of Multiple Myeloma: Prognostic Factors and Outcome..JCR 2016;6:247-250 |
|
Ahmad Malik A, Sereihan A, Iqbal JMalignant Pleural Effusion of Multiple Myeloma: Prognostic Factors and Outcome..JCR [serial online] 2016[cited 2025 Dec 17];6:247-250. Available from: http://www.casereports.in/articles/6/2/Malignant-Pleural-Effusion-of-Multiple-Myeloma.html |

|
|
|
|
|