Introduction
The CT-guided percutaneous biopsy is an established procedure in various regions of the body. In general, however, uterine or ovarian lesions rarely become candidates for percutaneous biopsy. Especially in the diagnosis of uterine lesions, endometrial brushing cytology and endometrial biopsy are widely performed, percutaneous uterine biopsies are rarely performed, and there are few reports about CT-guided biopsy in uterine lesions.
We performed CT-guided percutaneous biopsy for gynecological lesions including uterus and ovaries, and obtained sufficient materials in three patients for whom endometrial brushing cytology and endometrial biopsy were not diagnostic. In these cases, histological diagnoses from the biopsy contributed to the treatment strategy decision. Because the procedures seemed to be possibly useful diagnostic options that may become the standard for open biopsy by laparotomy, herein we report these 3 cases with a literature review.
Case Reports
Case 1
A 26-year-old woman was admitted to our hospital with a chief complaint of hypermenorrhea over a period of several months. She had experienced menstrual irregularity since she was a junior high school student. She had experienced repeated metrorrhagia in the year previous to her admission, and had a blood transfusion six months prior to admission due to anemia. She was admitted to our hospital for a close inspection of her anemia.
Laboratory data showed no abnormality in blood count or biochemistry. As for tumor markers, her CA125 level was 72.1 U/mL and her estradiol level was 658.9 pg/mL. An MR image of her pelvis showed an enlarged uterus. A T2-weighted image showed a hyperintense mass in both the endometrium and myometrium of the anterior wall of the uterine body [Fig.1a]. Multiple small cystic lesions were included within the lesion. The junctional zone neighboring the lesion became indistinct. There were no distinct hemorrhages or diffusion restrictions within the lesion. The MR images led to differential diagnoses of atypical adenomyosis, low-grade endometrial stromal sarcoma (ESS), and malignant mixed Mullerian tumor (MMMT).
Uterine cervical cytology, endometrial brushing cytology, and endometrial biopsy were performed, but a histological examination showed no evidence of a malignant finding. However, we attempted a CT-guided biopsy because, generally, it is reported that the diagnosis rate of endometrial biopsy in ESS is low. In this case, a CT-guided biopsy was performed by the coaxial method using a 16/18G Quick-Core biopsy needle from an anterior abdominal wall with the patient in a supine position. After the lesion in the anterior wall of the uterus was confirmed on unenhanced CT with reference to the MR images, biopsy specimens were obtained from three different places of the lesion using fluoroscopic guidance [Fig.1b].
The histopathological examination showed no evidence of malignancy, but extensive hyperplasia of the endometrial tissue was confirmed, indicating adenomyosis. Clinically, the lesion was diagnosed as adenomyosis that caused pseudowidening in conjunction with the MR images. Considering the results of the biopsy, hormonal therapy using GnRH analog administration was started, after which she immediately stopped menstruating, and her hypermenorrhea improved. Two months later, the levels of CA125 and estradiol had decreased to 5.0 pg/mL and 17.5 U/mL, respectively. MR images taken 6 months after the hormonal therapy showed remarkable reduction of the uterine mass and improvement of the uterine enlargement [Fig.1c].
Case 2
A 56-year-old woman admitted to our hospital for a lower abdominal mass. She had noticed the mass and increased urinary frequency one month earlier. So she came to our hospital for a close inspection of the symptoms. She had a cervical canal duct reefing more than 10 years earlier.
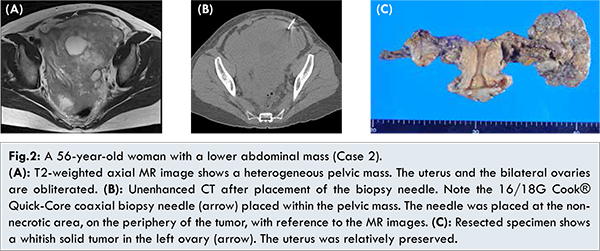
Laboratory data showed no abnormality in her blood count or biochemistry. As for tumor markers, the levels of CEA and CA125 were in the normal ranges. A body CT image showed a bulky pelvic mass occupying a pelvic cavity. Lymph node metastases in the bilateral supraclavicular fossa, mediastinum, and para-aortic region were found to be associated with this mass. Peritoneal dissemination was also detected. A T2-weighted image in the pelvis showed a large hyperintense mass involving the uterus and showing extensive diffusion restriction as well as significant contrast enhancement [Fig.2a]. The lesion also showed internal necrosis. The uterus and the ovaries were gathered in clusters and were difficult to isolate.
Although the imaging studies strongly suggested pelvic malignancies, it was not clear in which organ the malignancies had originated. Because of pain due to the adhesion at the uterine cervix, it was difficult to perform a histological diagnosis via the transvaginal uterine endometrial biopsy. A CT-guided biopsy was attempted for the treatment strategy decision. In this case, a CT-guided biopsy was performed by the coaxial method using a 16/18G Quick-Core biopsy needle from an anterior abdominal wall with the patient in a supine position. Under CT fluoroscopic guidance, tumor specimens were obtained from parts that had little hemorrhaging or necrosis by referring to MR images [Fig.2b]. The histopathological examination led to a diagnosis of adenocarcinoma, and immunochemical staining demonstrated that the cancer had a Mullerian duct origin, as suggested by the positive findings of PAX8 and the negative findings of GATE. Significant reduction in tumor size was confirmed on CT and MRI after systemic chemotherapy (adriamycin and cisplatin (AP) therapy followed by paclitaxel and carboplatin (TC) therapy because of drug eruption). Considering the patient’s clinical course, we considered that the reduction surgery was possible, and performed abdominal total hysterectomy, bilateral adnexectomy, retroperitoneal lymph node dissection, periaortic lymphadenectomy, and omentectomy. Intraoperative findings showed a 7.7 cm whitish solid mass in the left ovary tumor and that the tumors surrounding the uterine tumor were resolved [Fig. 2c]. The pathological diagnosis of the resected specimen was serous adenocarcinoma of the ovary. Finally, this case was considered to have involved the uterus from the left ovarian cancer.
A 70-year-old woman was admitted to our hospital for abdominal distension. She had noticed the distension 2 months earlier, and ascitic fluid had been detected by ultrasound in a medical check-up conducted at that time. She then came to our hospital for a close inspection of the symptoms. Her obstetrical history was significant, as it included a cesarean section. She had no significant medical history.
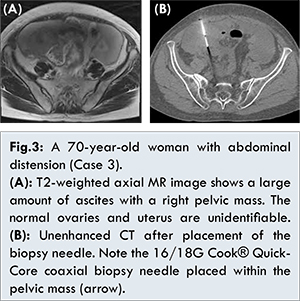
Laboratory data showed no abnormality in her blood count or biochemistry. As for tumor markers, her CA125 level was 598 U/mL while her CEA and CA19-9 levels were within the normal range. In imaging studies, a body CT image showed a 7 cm mass on the right side of the pelvic cavity, as well as lymph node metastases in the mediastinum and para-aortic region, peritoneal dissemination, and bone metastasis to the coccyx. A T2-weighted MR image of the pelvis showed a lobulated hyperintense mass with extensive diffusion restriction and significant contrast enhancement [Fig.3a].
The imaging findings strongly suggested a malignant tumor, but ascites fluid cytology produced no malignant findings. CT-guided biopsy for the lower pelvic mass was attempted for the treatment strategy decision. In this case, CT-guided biopsy was performed by the coaxial method using a 16/18G Quick-Core biopsy needle from an anterior abdominal wall with the patient in a supine position under an IVR-CT device [Fig.3b]. The pathological diagnosis was small cell carcinoma. Systemic chemotherapy was subsequently started. Although the tumor had decreased after three courses of systemic chemotherapy using paclitaxel and carboplatin (TC) therapy, exacerbation of the peritoneal dissemination and bone metastases of the thoracolumbar spines occurred. Therefore, supracervical hysterectomy, bilateral adnexectomy, resection of peritoneal implants, omentectomy, and intraperitoneal port placement were attempted. Intraoperative findings showed a 3 cm mass in the right ovary in conjunction with the small implants of the right retroperitoneal space. Intraoperative findings also showed an omental cake and a large number of peritoneal disseminations with the large, 4 cm diameter implant in Douglas’ fossa. The exacerbation of lymph node metastases was detected on CT 3 months after the surgery, after which chemotherapy using cisplatin and irinotecan (CDDP and CPT-11) was started as a postoperative systemic chemotherapy. After four courses of chemotherapy, there is no evidence of exacerbation of the metastasis.
Generally, the advantage of CT-guided pelvis tumor biopsy is low invasiveness compared with laparotomy, while the disadvantages are small size of the biopsy specimen and the inability to macroscopically confirm the pelvic mass itself. Yarram et al. [
1] performed ultrasound and CT-guided tumor biopsy for 111 cases of malignant pelvic tumors, and reported that there was no significant difference in the results between CT-guided biopsy (technical success rate, 93.2%; proper diagnostic rate, 90.2%; sensitivity, 84.6%; and specificity, 100%) and ultrasound guided biopsy (technical success rate, 97.0%; proper diagnostic rate, 95.4%; sensitivity, 94.5%; and specificity 100%). Both biopsy procedures have excellent diagnosability and low complication rates. However, most of these cases are postoperative pelvic recurrences or lymph node metastases of gastrointestinal cancers. There were only 2 cases (endometrial cancer and granulosa cell tumor in each) in which primary tumors of the uterus and the ovary were diagnosed by percutaneous biopsy. Because in our 3 cases the organs of origination and the etiology were unknown in the histological diagnosis by transvaginal biopsy, a percutaneous biopsy was performed [Table 1]. Ovarian tumors can be candidates for percutaneous tumor biopsy conventionally, and there are few reported cases in which a diagnosis was based on percutaneous needle biopsy. That is because in ovarian tumors there is a risk of peritoneal dissemination due to needle biopsy. Gupta et al. [
3] performed percutaneous CT-guided biopsy via the iliopsoas muscle for 5 cases of ovarian tumors without any complications. However, we do not seem to serve as a reference about there not having been peritoneal dissemination in the 5 cases because most of those cases were benign tumors. However, Thabet et al. [
4] performed image-guided percutaneous biopsy in 27 patients with ovarian tumors without any complications: in the cases of 9 patients with ovarian cancer, peritoneal dissemination was not detected after the biopsy after an average follow-up period of 44.6 months. Even if our 2 cases are considered together, there are few cases with percutaneous biopsy of ovarian tumor. However, it seems likely that cases will appear for which an effective chemotherapeutic regimen will be determined by the results of histologic specimens or cases that already show peritoneal dissemination on the initial images.
For the histological diagnosis of uterine masses, transvaginal cervical cytology and uterine endometrial biopsy are in widespread use. However, in cases with suspected uterine sarcoma, it was reported that sensitivity in uterine endometrial biopsy is low and that transcervical canal needle biopsy for the extra-uterine component of the tumor is useful [
5,
6]. At present, the indication for percutaneous uterine tumor biopsy is hard to discuss because there are so few cases. However, on the basis of our cases, cases in which malignancy cannot be completely denied, particularly cases that already present peritoneal dissemination seem to be good indications for this examination. There are some points to be kept in mind while performing a percutaneous uterine biopsy. First, the myometrium is originally plethoric, and the risk of bleeding cannot be completely eliminated. Then, the tumor seeding along the puncture course is also important. Therefore, we discuss thoroughly the risks and benefits of biopsy, including the presence of bleeding tendency, the approach course, and the policy with regard to bleeding among the teams of departments of obstetrics and gynecology and radiology. Percutaneous biopsy is then determined after sufficient explanation to, and the consent of, the patients.
In summary, CT-guided percutaneous biopsies of the uterus and ovaries can be performed safely by using three-dimensional reconstructed CT images and CT fluoroscopy, and sufficient histopathological specimens can be certainly obtained for diagnosis, producing useful information for treatment strategy decisions.
References
- Yarram SG, Nghiem HV, Higgins E, Fox G, Nan B, Francis IR. Evaluation of imaging-guided core biopsy of pelvic masses. Am J Roentgenol. 2007;188(5):1208-1211.
- Eriksson O, Hagmar B, Ryd W. Effects of fine-needle aspiration and other biopsy procedures on tumor dissemination in mice. Cancer. 1984;54(1):73-78.
- Gupta N, Rajwanshi A, Dhaliwal LK, Dey P, Srinivasan R, Nijhawan R. Fine needle aspiration cytology in ovarian lesions: an institutional experience of 584 cases. Cytopathology. 2012;23(5):300-307.
- Thabet A, Somarouthu B, Oliva E, Gervais DA, Hahn PF, Lee SI. Image-guided ovarian mass biopsy: efficacy and safety. J Vasc Interv Radiol. 2014;25(12):1922-1927.
- Yoshida C, Ichimura T, Kawamura N, Nakano A, Kasai M, Sumi T, Ishiko O. A scoring system for histopathologic and immunohistochemical evaluations of uterine leiomyosarcomas. Oncol Rep. 2009;22(4):725-731.
- Kawamura N, Ichimura T, Ito F, Shibata S, Takahashi K, et al. Transcervical needle biopsy for the differential diagnosis between uterine sarcoma and leiomyoma. Cancer. 2002;94:1713-1720.