Introduction
HAV infection remains either asymptomatic or resolves with mild symptoms without development of chronic disease. However, once it occurs as a co-infection with other viruses, it may develop serious disease [
1]. Management of hepatitis A may present several diagnostic challenges. This is a case of report 22 year old male patient with biochemical, serological, hematological and histopathological evidence of underlying Wilson’s disease complicated by hepatitis A virus infection. In our case the patient developed acute hepatic decompensation and it indicates that HAV plays a part in the acute hepatic decompensation seen in cases of unrecognized Wilson’s disease. Also persistent asymptomatic elevation of liver enzymes, the presence of hemolytic anemia in conjunction with the hepatic dysfunction, or elevation of unconjugated (indirect) bilirubin, should warrant the clinician to the possibility of Wilson’s disease. This can lead to early intervention to avoid progressive development of cirrhosis and fulminant liver failure.
Case Report
22 year male with no prior history of any disease consulted his doctor because of abdominal pain, generalized weakness, fever, yellow discoloration and repeated vomiting episodes for 5 days. There was no history suggestive of copper intoxication and dietary intake of copper was normal and other family members did not had any known history of liver disease.
On general physical examination, he had fever, moderate icterus, yellowish discoloration of the skin and nails [Fig.1]. On abdominal examination, firm hepatosplenomegaly was present. Abdominal ultrasound demonstrated moderate hepatosplenomegaly and retroperitoneal lymphadenopathy. Ophthalmologist identified classic Kayser-Fiersher rings on slit lamp examination.
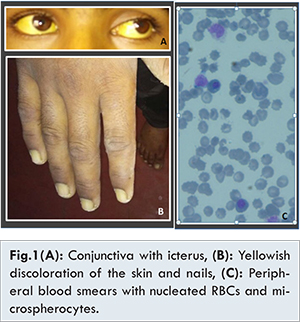
Hematological examinations reveled anemia [hemoglobin: 6.05 gm/dL (n=12-15 gm/dL), MCV: 96.7 (n=80-100 fl), MCH: 38.3 (27-33 pg/cell), MCHC: 39.6 (n=32-36 g/dL), RDWcv: 17.2 (n=11.5-14.5%)] with slight anisocytosis and poikilocytosis. There was leukocytosis with shift to left in the myeloid lineage up to myelocyte [Total WBC count 66.6 x 103 (n=4-11x 103/cu.mm)], with differential count as myelocyte: 5%, metamylocyte: 5%, band forms: 7%, neutrophils: 42%, lymphocyte: 15%, eosinophil: 3%, monocyte 6%, normoblast: 17%.), platelets were adequate [(platelets: 247x 103/cmm (n=150-450 x 103/cu.mm)] and no parasites were detected on the peripheral blood smear examination.
The peripheral smear also had 17% of normoblast and spherocytosis indicative of hemolytic anemia, which was further confirmed by serum biochemistry. Serum biochemistry investigations reveled deranged liver function [Total serum bilirubin of 34.6 mg/dL (n=0.2-1.2 mg%), direct bilirubin of 12.8 (n=upto 0.4 mg%), and indirect bilirubin of 21.8 (n=upto 0.8 mg%)], elevated liver enzymes, [SGOT: 62 (n=0-45), SGPT: 48 (n=0-48)]. Serum LDH levels were elevated markedly [8545.6 U/L, (n= 135-225U/L)]. Hematological investigations for direct and indirect Coomb’s test performed by gel technology were negative.
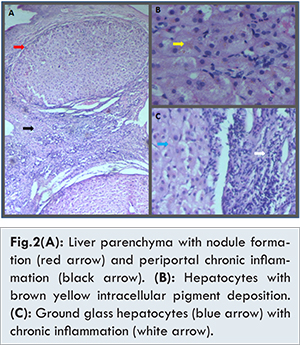
Serological tests for anti HAV IgM were positive (ELISA), while hepatitis B (HBsAg Virucheck), hepatitis E, human immunodeficiency virus I and II (HIV Tridot), IgM for dengue fever were negative. There were no serological features of autoimmune hepatitis (anti-nuclear antibody negative). Serum copper levels performed by biochemical methods were high 203.8 mcg/dL (n=70-140 mcg/dL). Serum ceruloplasmin levels turned out to be 56.21 mg/dL (n=15-30 mg/dL) done by immunoturbiditometric method. Urine copper levels in 24 hours urine specimen determined by atomic absorption graphite furnace method were elevated to 324 µg/24 hours (24 hours urine volume 3600 ml). Core biopsy of the liver was obtained. Microscopic examination revealed distortion of basic architecture in liver tissue. Liver parenchyma showed nodule formation surrounded by fibrosis and chronic inflammation. Hepatocytes showed micro vesicular steatosis, and intracellular yellowish brown pigment accumulation. There was no iron deposition [Fig.2].
Considering the clinical presentation, positive hepatitis A antibody, high serum and urinary copper, presence of Kayser-Fleischer ring with a background of a hemolytic anemia, diagnosis of a hepatitis A superimposed on Wilson’s disease was established. The patient was hospitalized and the routine treatment for severe hepatic dysfunction was initiated, including oxygen support, lactulose, oral/systemic antibiotic therapy, fluid and electrolyte management. A total of three cycles of plasmapheresis were carried out. Thereafter, serum copper and liver enzymes levels declined markedly. Moreover, the serum LDH and total bilirubin also decreased after the treatment. Peripheral blood smear also showed decrease in the spherocytes and nucleated RBCs.
Discussion
Although hepatic dysfunction in the Wilson’s disease may assume several forms, the symptoms could be nonspecific and can be mistaken for viral hepatitis. In our case patient presented with jaundice, hemolytic anemia and deranged liver function. Persistent asymptomatic elevation of liver enzymes, the presence of hemolytic anemia in conjunction with the hepatic dysfunction, or elevation of unconjugated (indirect) bilirubin, should warrant the clinician to the possibility of Wilson’s disease.
Although acute viral hepatitis due to hepatitis A virus (HAV) is documented to have a mild illness with a benign outcome it is often symptomatic in adults and a fulminant form is seen infrequently. The factors which may result in severe course of hepatitis A are found to be correlated with factors like older age and presence of underlying chronic liver disease. In a study done by Keeffe et al. hepatitis A superimposed on chronic liver disease is associated with high rate of morbidity and mortality [
2]. In our case coexistence of these two conditions certainly contributed to the severe damage to the liver and presentation of the acute liver failure and is a prototype example proving this theory. Similar findings were also described by Özçay et al. in their case report [
3]. So it is very crucial to investigate underlying chronic liver disease such as a Wilson’s disease in appropriate clinical setting of hemolytic anemia, liver dysfunction. A timely diagnosis and prompt treatment with the plasmapheresis can be life-saving. In a case report by Balkema et al. [
4] and Santra et al. [
5], they described hemolytic anemia as a first sign of Wilson’s disease and so was in our case. Sallie R et al. [
6] reported fulminant hepatic failure resulting from coexistent Wilson’s disease and hepatitis E and according to them viral infection may play a part in the acute decompensation of the liver function.
In our case the serum ceruloplasmin levels were elevated which are typically found to be decreased in the setting of a Wilson’s disease. As ceruloplasmin is an acute phase reactant it can be increased in the setting of inflammation, tissue injury, acute infection. In this particular case the overt hepatitis A super-infection could be the possible cause for the elevated ceruloplasmin levels in setting of underlying Wilson’s disease [
8]. Mohammad Reza Pashaei et al. [
9] in their case report suggested that plasmapheresis may be a lifesaving intervention during fulminant hepatic failure of Wilson’s disease. In our case indeed patient improved after 3 cycles of plasmapheresis.
Conclusion
Recently some studies from India have reported an increase in symptomatic cases of HAV among older populations, so as to epidemiological shift, among the socio-economically developed population resulting in pockets of susceptible populations. This situation demands that relevant guidelines be evolved for HAV vaccination in these susceptible pockets and communities by characterizing them appropriately.
References
- Tandon BN, Gandhi BM, Joshi YK, Gupta H, Irshad M. Subclinical hepatitis A in north Indian children. The Lancet. 1984;11:335-336.
- Keeffe EB. Hepatitis A and B superimposed on Chronic Liver Disease: Vaccine-Preventable Disease. Trans Am Clin Climatol Assoc. 2006;117:227-228.
- Özçay F, Canan O, Akcan B, Bilezikçi B. Hepatitis A super infection as a cause of liver failure in a child with Wilson’s disease. Turk J Pediatr. 2007;49:199-202.
- Balkema S, Hamaker ME, Visser HPJ, Heine GDN, Beuers U. Haemolytic anaemia as a first sign of Wilson’s disease. Neth J Med. 2008;66:344-347.
- Santra G, Paul R, Choudhury PS, Ghosh SK, Dibyendu De, Das S. Haemolytic Anaemia as First Manifestation of Wilson’s Disease: A Report of Two Cases. JAPI. 2014;62:55-57.
- Sallie R, Chiyende J, Tan KC, Bradley D, Portmann R, Williams R, et al. Fulminant hepatic failure resulting from coexistent Wilson’s disease and hepatitis E. Gut. 1994;35:849-853.
- Strickland, GT’ Chang NK, Beckner WB. Hypersplenism in Wilson’s disease. Gut. 1972;13:220-224.
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369(9559):397-408.
- Pashaei MR, Ajdarkosh H, Daryani NE, Habibollahi P, Beigmohammadi MT. Life Saving Plasmapheresis for the Management of Hemolytic Crisis and Acute Liver Failure in Wilson’s disease. Hepatitis Monthly. 2009;9(2):154.