|
|
|
|
|
Role of Cetuximab in Pharyngocutaneous Fistula Closure due to Peristomal Recurrence
|
|
|
how much does abortion pill cost abortion facts
Departments of 1Otolaryngology, Head & Neck Surgery and 2Medical Oncology, Ministry of Health Diskapi Yildirim Beyazit Training and Research Hospital; 3Department of Otolaryngology, Head and Neck Surgery, Yildirim Beyazit University, Faculty of Medicine, Ankara, Turkey. |
|
|
|
|
|
Corresponding Author:
|
Dr. Ömer Bayir
Email: bayiromer@hotmail.com
|
|
|
|
|
|
|
|
|
Received:
26-JUL-2016 |
Accepted:
25-OCT-2016 |
Published Online:
20-DEC-2016 |
|
|
|
|
|
|
|
Abstract
|
|
|
|
Cetuximab is a monoclonal drug that has survival advantage in the treatment of patients with locoregionally advanced and platinum-refractory metastatic or recurrent head and neck squamous cell carcinoma (SCC). We report a case of 52 year-old man with peristomal recurrence of laryngeal SCC patient who was treated successfully with cetuximab, cisplatin and 5FU after organ preservation treatment and surgery. The patient presented with dysphonia and laryngeal lesions, underwent direct laryngoscopy (DL) with biopsies and the diagnosis was poorly differentiated SCC. Subsequently, he was treated with chemoradiation therapy. However, he developed hoarseness and dyspnea after chemoradiation. DL was performed due to transglottic lesion with suspicious left level 2A lymph node metastasis. Then, we performed total laryngectomy with neck dissection. Unfortunately, pharyngocutaneous fistula and peristomal recurrence developed after the surgery. The patient received palliative chemotherapy with cetuximab, cisplatin, and 5FU and acheived complete remission. Pharyngocutaneous fistula was closed with deltopectoral fasciocutaneous flap. No signs of recurrence were noted at 18 months followup. Concurrent therapy with cetuximab may be beneficial in patients with recurrent laryngeal cancer.
|
|
|
|
|
|
Keywords :
|
Biopsy, Cetuximab, Dyspnea, Laryngeal Neoplasms, Laryngoscopy.
|
|
|
|
|
|
|
|
|
|
|
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffa4e213000000fe01000001000a00 6go6ckt5b5idvals|700 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Cetuximab is a human-murine chimeric immunglobulin G1 monoclonal antibody which competitively binds to extracellular domain of the epidermal growth factor receptor (EGFR) [ 1]. It has a survival advantage in the treatment of patients with locoregionally advanced and platinum-refractory metastatic or recurrent head and neck squamous cell carcinoma (HNSCC) [ 2]. Combined cetuximab and chemotherapy or radiotherapy in recurrent or metastatic cancer is known to be effective in overall survival and progression free survival in different studies [ 3]. We present a case who had treated with cetuximab due to peristomal recurrence after chemoradiation and surgery.
Case Report
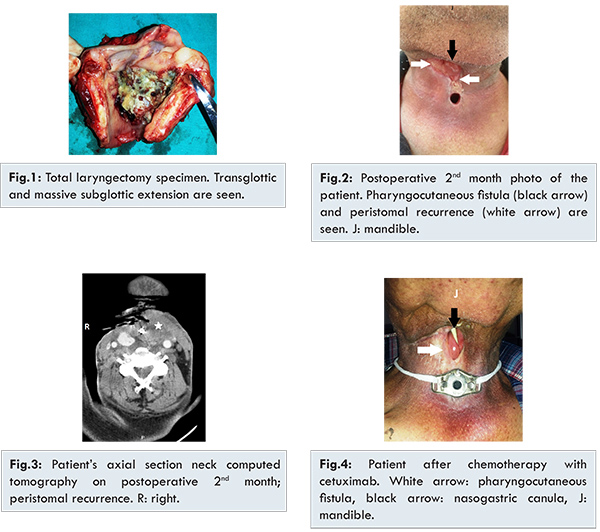
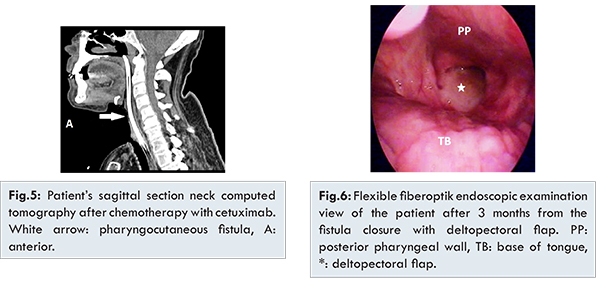
A 52 year old male patient was admitted to otolaryngology clinic due to dysphonia and laryngeal lesions. He underwent direct laryngoscopy with biopsies (DL) and the diagnosis was poorly differentiated SCC. Subsequently he underwent chemoradiation therapy (7 cycles, 75 mg/m2 cisplatin + 6300 Gy radiation) for T2N0M0 glottic laryngeal carcinoma. However, after three months of chemoradiation, he presented with hoarseness and dyspnea. DL was performed due to transglottic lesion with suspicious left level 2A lymph node metastasis. Then, we performed total laryngectomy with neck dissection [Fig.1]. The histopathological report was T4aN1M0 poorly differentiated SCC with 2 cm diameter extracapsulary invasion lymph node metastasis. Unfortunately, pharyngocutaneous fistula developed after two months of surgery. Thus, oral intake was stopped and nasogastric feeding was started. Open dressing and debridement were performed once a day for two weeks. Incisional biopsy was performed from the fistula’s margin due to the lack of fistula regression and the histopathologic report was peristomal recurrence of the laryngeal carcinoma [Fig.2,3]. After discussing the patient’s treatment options at our tumor board, the patient received palliative chemotherapy with 6 cycles, 75 mg/m2 cisplatin, 750 mg/m2 5-Fluorouracil , and 400 mg/m2 cetuximab (loading dose). After this therapy 250 mg/m2/week cetuximab (maintenance dose) was given for 3 months. At the end of the chemotherapy, only acneiform rash occurred on his skin which was treated with steroid cream. Incisional biopy was performed after 4 months which confirmed absence of malignancy. Neck computed tomography revealed totally tumor regression. The patient acheived histopathological and radiological remisssion [Fjg.4,5]. Pharyngocutaneous fistula was closed with deltopectoral fasciocutaneous flap and salivary bypass tube was applied and he started oral intake after the surgery [Fig.6]. The salivary bypass tube was removed 2 months after last surgery. No recurrence was seen after 18 months of palliative therapy.
Head and neck cancer is the sixth most common cancer worldwide. Unlike the other countries, larynx cancer is the most common head and neck cancer in Turkey [ 4,5]. Over the 500,000 new cases of HNSCC are diagnosed each year over the world [ 3, 6]. The management of HNSCC requires a multidisciplinary approach and it is challenging and complex process. Single modality treatment approaches (surgery or radiotherapy) are generally recommended for the early stages cancers. But, multimodality regimens are generally recommended for advanced stage cancers. Nowadays, concurrent cisplatin-based chemoradiation (CBCRT) is the standard approach for organ preservation without surgery and patients with unresectable disease [ 7]. CBCRT is also used for patients with high risk pathological findings at surgical resection. However, multimodality approaches are not often enough. Locoregional recurrence or distant methastasis (R/M-HNSCC) develop in significant number of the patients [ 1, 3, 8].
EGFR and its ligand (TGF-a) are overexpressed in the carcinogenesis in the vast majority of patients of HNSCC [9]. Overexpression of EGFR is related to poor prognostic factor in HNSCC [ 3, 10]. Recently, this situation is used in the treatment as EGFR targeting strategies for HNSCC; monoclonal antibodies and tyrosine kinase inhibitors [ 1, 10, 11]. The best studied monoclonal antibody for HNSCC is cetuximab. Cetuximab has been studied for locally advanced HNSCC patients [ 1- 3, 11]. But, we discuss cetuximab treatment for R/M-HNSCC in this presentation.
In ECOG trial (phase III randomized study), patients with R/M HNSCC were randomly assigned to receive cisplatin every 4 weeks, with weekly cetuximab or placebo [ 12]. The study concluded that the addition of cetuximab to cisplatin significantly improves response rate. Progression-free and overall survival were not significantly improved by the addition of cetuximab in this study. In EXTREME study, authors investigated the efficacy of cetuximab plus platinum-based chemotherapy as first-line treatment in patients with R/M-HNSCC [ 13]. They concluded that compared with platinum-based chemotherapy plus fluorouracil alone, cetuximab plus platinum-fluorouracil chemotherapy improved overall survival when given as first-line treatment. In the GORTEC 2008-3 trial, the efficacy and safety of four cycles of docetaxel associated with cisplatin and cetuximab followed by maintenance with cetuximab every 2 weeks were evaluated [ 14]. The study concluded that four cycles of docetaxel combined with cisplatin and cetuximab followed by maintenance cetuximab were feasible, favorable and precociously active with a manageable safety profile in fit patients with R/M-HNSCC.
The role of cetuximab in pretreated chemotherapy with platinum-refractory/resistant disease was evaluated in some studies [ 15- 17]. Overall survival and progression free survival were favorable in these studies. In R/M-HNSCC patients who progressed on platinum based chemotherapy, cetuximab may valuable option [ 1]. Recently, Ritter et al. demonstrated a second line new palliative treatment regimen, with organ preserving surgical debulking, followed by a combination of postoperative instertital brachytherapy and a simultaneous protocol of cetuximab and taxol. They concluded that pre-treated patients with a further relapse benefited from the cetuximmab-taxane recurrenc therapy. This new regimen improved overall survival and toxicicty was lower [ 18].
The vast majority of adverse effects of cetuximab related events are grade 1 and 2. Additionally, the most common adverse effect of cetuximab is rash which was occurred our patient. Fury et al. [19] studied two different doses of cetuximab in patients who had received no more than two prior cytotoxic chemotherapy regimens for R/M-SCCHN. Patients were treated with cetuximab every 2 weeks either at 500 mg/m² (Group A) or 750 mg/m² (Group B). Partial response rates were 11% for Group A and 8% for Group B. Progression free survival and overall survival rates were similar for both groups. In this study, the most common cetuximab-related adverse events included rash, fatigue, and hypomagnesemia. The others side effects of cetuximab are mucositis, xerostomia pain, dysphagia, performance status deterioration, hypomagnesemia, and weight loss [ 3]. However, serious side-effects develop and grade of side-effect gets worse when cetuximab is combined to cisplatin or radiotherapy [ 3, 12, 13].
In this presentation, patient was successfully treated with cetuximab and cisplatin after organ preservation and surgery. At the end of palliative therapy rash developed, which was treated with steroid cream. After 4 months from the palliative chemotherapy, patient’s fistula was closed with deltopectoral myocutaneous flap.
Conclusion
Appropriately selecting patients with R/M-HNSCC can benefit from cetuximab therapy in combination with palliative platine based chemotherapy. For advanced and recurrent laryngeal cancer cetuximab has to be in mind with dramatically good results.
References
- Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21 (suppl 7): vii252-vii261.
- Peddi P, Shi R, Nair B, Ampil F, Mills GM, Jafri SH. Cisplatin, Cetuximab, and Radiation in Locally Advanced Head and Neck Squamous Cell Cancer: A Retrospective Review. Clinical Medicine Insights: Oncology. 2015;9:1-7.
- Specenier P, Vermorken JB. Cetuximab: its unique place in head and neck cancer treatment. Biologics: Targets and Therapy. 2013;7:77-90.
- Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345(26):1890-1900.
- Gültekin M, Boztas G. (Ed.) Türkiye Kanser Istatistikleri 2009 yili verileri (Turkey Cancer Statistics 2009 data) (In Turkish). [Online]. Available from URL: http://kanser.gov.tr/Dosya/ca_istatistik/2009kanseraporu.pdf. Accessed on July 26, 2016.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
- Grégoire V, Lefebvre JL, Licitra L, Felip E. EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v184-v186.
- Specenier PM, Vermorken JB. Recurrent head and neck cancer: current treatment and future prospects. Expert Rev Anticancer Ther. 2008;8(3):375-391.
- Grandis JR. Established and emerging concepts in epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2007; 69(Suppl 2):S22-S24.
- Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170-4176.
- Peddi P, Shi R, Nair B, Ampil F, Mills GM, Jafri SH. Cisplatin, Cetuximab, and Radiation in locally advanced head and neck squamous Cell Cancer: a Retrospective Review. Clinical Medicine Insights: Oncology. 2015;9:1-7.
- Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23(24):8646-8654.
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116-1127.
- Guigay J, Fayette J, Dillies AF, Sire C, Kerger JN, Tennevet I, et al. Cetuximab, docetaxel, and cisplatin as first-line treatment in patients with recurrent or metastatic head and neck squamous cell carcinoma: a multicenter, phase II GORTEC study. Annals of Oncology. 2015;26:1941-1947.
- Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortés-Funes H, Hitt R, et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23(24):5568-5577.
- Herbst RS, Arquette M, Shin DM, Dicke K, Vokes EE, Azarnia N, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23(24):5578-5587.
- León X, Hitt R, Constenla M, Rocca A, Stupp R, Kovács AF, et al. A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol (R Coll Radiol). 2005;17(6):418-424.
- Ritter M, Teudt IU, Meyer JE, Schröder U, Kovács G, Wollenberg B. Second-line treatment of recurrent HNSCC: tumor debulking in combination with high-dose-rate brachytherapy and a simultaneous cetuximab-paclitaxel protocol. Radiat Oncol. 2016;11:6.
- Fury MG, Sherman E, Lisa D, Agarwal N, Algazy K, Brockstein B, et al. A randomized phase ii study of cetuximab every 2 weeks at either 500 or 750 mg/m2 for patients with recurrent or metastatic head and neck squamous cell cancer. J Natl Compr Canc Netw. 2012;10(11):1391-1398.
|
|
|
|
|
|
|
Search Google Scholar for
|
|
|
Article Statistics |
|
Saylam G, Bayir Ö, Inanç Imamoglu G, Altinbas M, Hakan Korkmaz MRole of Cetuximab in Pharyngocutaneous Fistula Closure due to Peristomal Recurrence.JCR 2016;6:586-591 |
|
Saylam G, Bayir Ö, Inanç Imamoglu G, Altinbas M, Hakan Korkmaz MRole of Cetuximab in Pharyngocutaneous Fistula Closure due to Peristomal Recurrence.JCR [serial online] 2016[cited 2025 Jun 6];6:586-591. Available from: http://www.casereports.in/articles/6/4/Role-of-Cetuximab-in-Pharyngocutaneous-Fistula-Closure-due-to-Peristomal-Recurrence.html |

|
|
|
|
|