Introduction
Corynebacterium diphtheriae (C. diphtheriae) is a leading upper respiratory pathogen responsible for a deadly disease of childhood, diphtheria. Despite increased immunization coverage it continues to play a lethal role in developing countries. India witnessed an initial decline in the incidence of diphtheria over the years with a total of only 1326 cases reported in 1997 [
1-
3]. However, in recent years, a resurgence of diphtheria has been observed where India has accounted for almost 80% (3,123) cases reported in the world (4,053) in 2010 [
4]. There have been several reports from various parts of the country demonstrating persistence [
5] and resurgence of disease with an age shift of occurrence from below five years to above the age of five years [
4,
6-
8]. Less commonly, the organism is also linked up with manifestations in the form of nasal, aural, conjunctival, cutaneous, umbilical and genital diphtheria due to the colonization of toxigenic C. diphtheriae strains in localized areas such as wounds, abscesses, skin lesions. At these sites, lesions usually occur as local pseudomembranous inflammation on mucosal surfaces accompanied by serosan-guineous exudates and exotoxin production followed by necrosis. Systemic lesions of the heart and (to a lesser extent) nerve due to potent exotoxin released by the bacterium increase the severity [
9]. According to the available literature, C. diphtheriae has been rarely implicated in causation of otitis media. Hereby, we report a rare case of isolation of toxigenic C. diphtheriae from a CSOM patient and discuss its role as a clinically significant and re-emerging pathogen.
Case Report
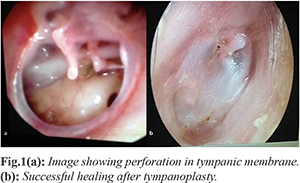
A 24 year female presented with pus discharge from left ear since childhood. The discharge was yellowish, intermittent, mucopurulent, non-foul smelling and non-blood stained. There was history of decreased hearing from left ear which was gradual in onset, progressive and subsequently interfering in routine communication. Patient had taken several courses of antimicrobial (oral and topical) treatment in the past to which she responded favourably every time. The patient’s general physical examination was within normal limits. On local examination, there was subtotal perforation with all margins present in the left ear
[Fig.1a] while, the right tympanic membrane was intact. There was no evidence of pseudomembrane or patch in the left middle ear. There was also no preauricular, postauricular or mastoid lymphadenopathy. Examination of right ear, nose and throat were within normal limits.
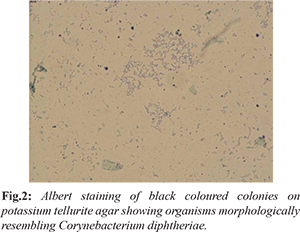
The pus discharge from left ear was collected with the help of a sterile swab stick and sent to microbiology laboratory for isolation of causative agent/s following which patient was offered empirical treatment with topical ciprofloxacin ear drops. Direct Gram staining of the sample showed presence of pus cells along with Gram positive bacilli that were thin, long, slightly curved with granules at both the poles and few were arranged in Chinese letter pattern resembling Corynebacterium spp. Few gram negative bacilli were also observed. Albert staining revealed rod like structures or bacilli with polar metachromatic granules resembling C. diphtheriae. On the basis of microscopic finding, culture was performed on blood agar, MacConkey’s agar and potassium tellurite medium. Plates were incubated at 370C and examined after 24 hours and 48 hours. After overnight incubation, growth suggestive of Pseudomonas aeruginosa and Klebsiella pneumoniae was observed. Potassium tellurite medium grew black coloured colonies after 48 hours of incubation which were identified as C. diphtheriae using Gram and Albert staining morphology
[Fig.2]. All the isolates were confirmed using standard biochemical tests [
10]. Pseudomonas aeruginosa was identified by its characteristic green pigment produced on the media, oxidase positivity, oxidative reaction in oxidative fermentative medium and motility. Klebsiella pneumoniae was identified by mucoid lactose fermenting colonies produced on MacConkey’s medium with negative oxidase reaction, positive Vogues Proskeur test, positive citrate utilization and urea hydrolysis test with negative methyl red test and absence of indole production. The isolate suspected as C. diphtheriae was sent to National Centre for Disease Control (NCDC), New Delhi for further confirmation and toxigenicity testing. It was confirmed as C. diphtheriae biotype mitis by Hiss’s serum sugars and other standard biochemical reactions and was reported to be positive for toxin production using Elek’s gel precipitation test. Meanwhile, patient underwent audiometry which showed moderately severe conductive hearing loss. X-ray mastoid (Schuller view) showed bilateral pneumatic mastoid.
Patient was planned for tympanoplasty. Type 1 tympanoplasty was done through post-aural route. In post-operative period amoxicillin was given for seven days. Treatment with an anti-diphtheritic serum was not warranted even after the positive toxigenicity report, due to virtual absence of signs of toxicity. At six weeks follow up, the tympanic graft was well accepted by host with good vascularity and no inflammation
[Fig.1b] and improved hearing. Patient was further followed up for six months during which there were no recurrence of symptoms. There were no children in the family or other close contacts of the patient. However, other familial contacts were examined and screened for nasopharyngeal carriage of C. diphtheriae by taking swabs for culture and sensitivity, which were reported as negative. They were further followed up for development of any diphtheria like illness. There were no such reports until six months of follow up.
Discussion
C. diphtheriae is an established upper respiratory pathogen. Increased immunization coverage in childhood was able to decrease the incidence of diphtheria initially. However, resurgence is being reported from both developed and developing countries like India which warrants serious concern [
11]. India follows Universal Immunization Program where it offers three doses of DwPT at six, ten and fourteen weeks and two booster doses at 16-24?months and five years of age. Over 94-100% children develop protective levels of antibodies following primary immunization but in the absence of boosters the titres drop and over 20-65% of adults may become susceptible [
12]. The reappearance of diphtheria in a milder form and dominantly affecting older children or adults owes to, reduced opportunity to acquire natural immunity due to successful childhood vaccination programs and waning of previously acquired immunity in the absence of natural boosting/adult vaccination program [
3]. Furthermore, vaccination using diphtheria toxoid targets bacterial toxin and not the infecting organisms which also carry the property for colonization and invasion. This has led to the situation where localized infections are increasingly being reported, with no systemic manifestations or that caused by non-toxigenic strains of C. diphtheriae [
13-
15].
Otitis media is a localized lesion with possible acquisition of infecting organisms either from nasopharynx or from external ear canal. An association of C. diphtheriae with otitis media is relatively rare phenomenon which is generally seen accompanying diphtheritic croup and nasopharyngitis causing complications like destruction of tympanic membrane and ossicles, invasive infection of the contiguous structures leading to necrosis of the mastoid process, temporal bone and labyrinth [
9]. The three varieties of diphtheritic otitis media that have been reported are, i) primary diphtheritic otitis media, where middle ear is the main site of infection with isolation of organisms from ear discharge and not from the nasopharynx; ii) secondary diphtheritic otitis media where ear is involved as a complication of preexisting nasopharyngeal diphtheria and obvious positive cultures obtained from the ear and nasopharynx; and iii) superimposed infection by C. diphtheriae upon an existing otitis media due to the usual respiratory pathogens like streptococci, pneumococci or Haemophilus infleuenzae [
16]. Primary diphtheritic otitis media is rare and has been reported in few old studies [
16-
19]. In the case presented here, the organism was isolated in addition to organisms like Pseudomonas aeruginosa and Klebsiella pneumoniae which are the potential pathogens associated with CSOM. Also, there was no history of previous illness suggesting diphtheria. This confirms the entity to be the third variety of diphtheritic infection i.e. a superimposed infection by C. diphtheriae. Superimposed infection by C. diphtheriae in otitis media patients suffering from primary disease due to known pathogens like haemolytic Streptococci and S. aureus has been reported as a predominant variety in the literature [
19-
20]. Dixon et al. (1984) [
20] also signified the role of endemic cutaneous diphtherial lesions as a possible source of superimposed infection. Although we could not find any cutaneous lesion nor do the nasopharyngeal cultures revealed C. diphtheria. Moreover, in addition the possibility of previous nasopharyngeal colonization resulting in access to middle ear during the bouts of upper respiratory tract infections cannot be overlooked, especially in the era of resurgence of diphtheria as mentioned above.
From a clinical standpoint, the only difference that may distinguish C. diphtheriae from other organisms in ear is pseudomembrane formation. However, this is found in only 23% of reported cases as per the review by Downes et al. (1959) [
18]. Hence bacteriological confirmation is important. It has also been speculated that toxigenic as well as non-toxigenic strains [19-20] of C. diphtheriae are equally capable of causing diphtheritic otitis media leading to an equal proportion of pyogenic complications. Toxigenic strains can lead to serious systemic manifestations warranting treatment with antitoxin (ADS). However, in the present case despite isolation of ‘toxigenic’ C. diphtheriae, systemic toxicity was not evident. Hence, treatment with antitoxin was not given. Absence of systemic toxicity in the patient might be because of in vivo neutralization of the produced toxin by circulating antitoxin antibodies in patient’s serum. This is supplanted by the fact that the patient was a young adult with history of complete immunization in childhood implying presence of protective level of circulating antitoxin. However, exact level of circulating antitoxin in the patient (protective antbody levels =0.1 IU/ml) [
21] was not determined in this case.
Determining antitoxin level against diphtheria toxoid is a parameter which is not practiced in a routine diagnostic laboratory until it is specifically requested. Hence, most of the diagnostic laboratories in India lack ready availability of serological kits determining diphtheria antitoxin levels. Likewise, the facility for quantitative estimation of diphtheria antitoxin level was unavailable in our hospital also at the time of presentation of this patient. In this geographical region, it was the first case warranting estimation of antibody levels followed by identification of few more similar cases (unpublished data), till the submission of this report. Given a situation and possible presence of carriers/unreported cases in the local population, attempts for procurement and constant availability of serological kits for antibody level estimation have been initiated.
While immunity due to vaccination may be considered as a more logical explanation for the absence of systemic toxicity in the patient; it does not preclude existence of other possible explanations. As evident from few studies, toxin production does not always correlate with the pathogenicity, since non-toxigenic strains are equally seen to be contributing to the harmful effects and severe disease [
15,
22]. On the other hand, organism carrying the toxin gene may not always express its toxin [
23]. One limitation in our case, though it did not affect the final result but definitely delayed the interpretation, was that we could not inoculate the specimen on Loeffler’s serum slope as per the routine protocol. This was because we had received only one swab for testing. Through this report we want to draw the attention to the fact that for any specimen where swabs are sent for investigation, the clinicians must ensure that they send them in duplicate so that proper laboratory protocols can be followed.
Conclusion
Isolation of cases with C. diphtheriae as a sole pathogen of middle ear should demand an insight into the pathogenic mechanisms involved. In addition, environmental shedding of the organism may pose an unseen threat of emergence of fatal diphtheria in the contacts and other susceptible local population. Hence, active screening for carriage of C. diphtheriae as an aural colonizer/pathogen is advisable especially in the areas where resurgence of diphtheria is expected. This should also be done in adults or elderly individuals wherein the level of protection by immunization has been lowered to a level that individuals become susceptible to toxigenic strains of C. diphtheriae despite complete immunization.
Acknowledgement: National Centre for Disease Control (NCDC), New Delhi, India for providing valuable support in identification and toxigenicity testing of the C. diphtheriae isolate.
Contributors: UG, NG: Study conception and critical revision; RA: Patient management and manuscript revision; AB, PD, SSN, SK: Critical revision. UG will act as guarantor. All authors approved the final version of the paper.
Funding: None;
Competing interests: None stated.
References
- World Health Organization. Chronic suppurative otitis media: burden of illness and management options, Geneva:WHO. 2004. Available from: http://www.who.int/pbd/publications/Chronicsuppurativeotitis_media.pdf. Accessed on September 13, 2016.
- Massa HM, Cripps AW, Lehmann D. Otitis media: viruses, bacteria, biofilms and vaccines. Med J Aust. 2009;191(9 Suppl): S44-49.
- Singhal T, Lodha R, Kapil A, Jain Y, Kabra SK. Diphtheria-down but not out. Indian Pediatr. 2000;37(7):728-738.
- Dandinarasaiah M, Vikram BK, Krishnamurthy N, Chetan AC, Jain A. Diphtheria re-emergence: Problems faced by developing countries. Indian J Otolaryngol Head Neck Surg. 2013;65(4):314-318.
- Bhagat S, Grover SS, Gupta N, Roy RD, Khare S. Persistence of Corynebacterium diphtheriae in Delhi & National Capital Region (NCR). Indian J Med Res. 2015;142(4):459-461.
- Sharma NC, Banavaliker JN, Ranjan R, Kumar R. Bacteriological & epidemiological characteristics of diphtheria cases in & around Delhi a retrospective study. Indian J Med Res. 2007;126(6):545-552.
- Dravid MN, Joshi SA. Resurgence of diphtheria in Malegaon and Dhule regions of north Maharashtra. Indian J Med Res. 2008;127(6):616-617.
- Khan N, Shastri J, Aigal U, Doctor B. Resurgence of diphtheria in the vaccination era. Indian J Med Microbiol. 2007;25(4):434.
- Bluestone CD, Klein JO. Otitis Media in infants and Children, 2nd edn. WB Saunders Company; 1995.
- Koneman EW, Allen SD, Janda WM, Shreckenberger PC, Winn WC. Colour atlas and textbook of diagnostic microbiology, 5th edn., Philadelphia: Lippincott-Raven Publishers; 1997.
- John TJ. Resurgence of diphtheria in India in the 21st century. Indian J Med Res. 2008;128(5):669-670.
- Phalkey RK, Bhosale RV, Joshi AP, Wakchoure SS, Tambe MP, Awate P, et al. Preventing the preventable through effective surveillance: the case of diphtheria in a rural district of Maharashtra, India. BMC Public Health. 2013;13:317.
- Hadfield TL, McEvoy P, Polotsky Y, Tzinserling VA, Yakovlev AA. The pathology of diphtheria. J Infect Dis. 2000;181(Suppl 1): S116-20.
- Edwards B, Hunt AC, Hoskisson PA. Recent cases of non-toxigenic Corynebacterium diphtheriae in Scotland: justification for continued surveillance. J Med Microbiol. 2011;60:561-562.
- Kanungo R, Vijayalakshmi N, Nalini P, Bhattacharya S. Diphtheria due to non-toxigenic Corynebacterium diphtheriae: A report of two cases. Indian J Med Microbiol. 2002;20:50-52.
- Revathi G and Goyal A. Primary diphtheritic otitis media and mastoiditis. Indian J Otolaryngol Head Neck Surg. 1998;50(2):178-180.
- Bane WC. Acute otitis media of diphtheritic origin. The Laryngoscope. 1917;27:626-629.
- Downes JJ. Primary diphtheritic otitis media: review of the literature and report of a case. AMA Arch Otolaryngol. 1959;70(1):27-31.
- Jellard CH. Diphtheria infection in North West Canada, 1969, 1970 and 1971. J Hyg (Lond). 1972;70(3):503-510.
- Dixon JMS. Diphtheria in North America. J Hyg(Lond). 1984;93:419-432.
- Bergamini M, Fabrizi P, Pagani S, Grilli A, Severini R, Contini C. Evidence of increased carriage of Corynebacterium spp. in healthy individuals with low antibody titres against diphtheria toxoid. Epidemiol Infect. 2000;125(1):105-112.
- Lake JA, Ehrhardt MJ, Suchi M, Chun RH, Willoughby RE. A case of necrotizing epiglottitis due to nontoxigenic Corynebacterium diphtheriae. Pediatrics. 2015;136(1): 242-245.
- Zakikhany K, Neal S, Efstratiou A. Emergence and molecular characterisation of non-toxigenic tox gene-bearing Corynebacterium diphtheriae biovar mitis in the United Kingdom, 2003-2012. Euro Surveill. 2014;19(22): pii:20819.
- Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology. 7th ed. Elsevier Saunders; 2012.
- Todar K. Diphtheria. Todar’s online Textbook of Bacteriology; 2004. Available from http://www.textbookofbacteriology.net/diphtheria_3.html. Accesssed on September 13, 2016.