6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffe45a1a0000002206000001000100
6go6ckt5b5idvals|767
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Oligodendroglioma’s (ODG) are primary glial brain tumors that are divided into classical ODG’s [World Health Organization (WHO) grade II]and anaplastic ODG’s (WHO grade III). The corpus callosum is the largest commissure of the brain consisting of tightly packed white matter tracts connecting the two cerebral hemispheres. Common pathologies encountered in this region include primary CNS lymphomas and glioblastoma multiforme [1].
Case Report
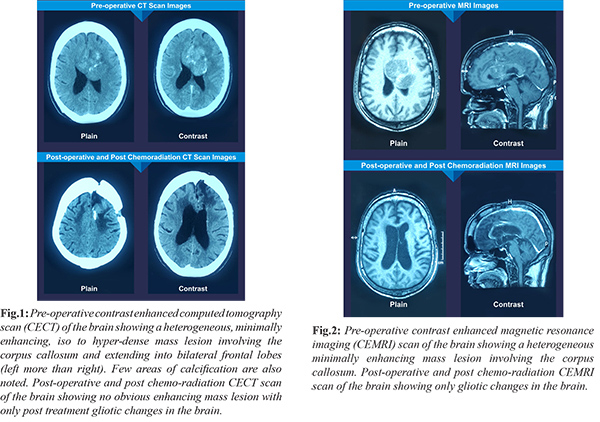
A 54 year old male was admitted with complaints of intermittent holo-cranial headache since six months not associated with nausea, vomiting or any visual symptoms. Patient also complained of gradually progressive weakness of right side of the body since four months. On examination, he was conscious and oriented. He had grade 4/5 power in right upper and lower limbs. He had bilateral papilledema. Tests for human immunodeficiency virus (HIV), hepatitis B surface antigen (HBsAg) and hepatitis C virus were negative. Contrast enhanced computed tomography (CECT) scan and contrast enhanced magnetic resonance imaging (CEMRI) scan of the brain were suggestive of a heterogeneous minimally enhancing mass arising from the corpus callosum and extending bilaterally into the frontal lobes (left more than right). Few areas of calcification were also noted [Fig.1,2].
Patient was taken up for left frontal craniotomy and tumor decompression. On histo-pathological examination, sheets and papillaroid clusters of monomorphic tumor cells, showing classical oligodendroglial like morphology, were seen. The tumor cells classically demonstrated perinuclear halos giving a “fried egg appearance”. Mini-gemistocytes were also seen. The tumor cells were negative for glial fibrillary acidic protein (GFAP) staining. The MIB index was 5-7% [Fig.3]. Significant number of tumor cells showed combined 1p and 19q deletions. The final histopathological report was “oligodendroglioma grade II but likely to behave in an aggressive manner”.
Post-surgery, patient received adjuvant concurrent chemo-radiation followed by 18 cycles of oral temozolomide chemotherapy. On his latest follow up, 2 years post-surgery, CECT and CEMRI scans of the brain showed no obvious enhancing mass lesion but only post treatment gliotic changes in the brain [Fig.1,2].
Discussion
Oligodendroglioma is believed to originate from the oligodendrocytes of the brain or from a glial precursor cell. These tumors occur in both sexes, with a male: female ratio of 2:1. There are two peaks of incidence: between the age of 6 to 12 years in children and between the age of 35 to 44 years in adults [2]. More than 90% of ODG’s arise in the supra-tentorial white matter, most commonly in the frontal and temporal lobes. Less than 10% occur in the posterior fossa and spinal cord [2]. ODG’s typically demonstrate a slow, infiltrative growth pattern. Cerebrospinal fluid (CSF) metastasis reportedly occurs in upto 14% of the cases [3,4].
In these patients, seizures are the presenting symptom in approximately 50-80% of the cases [3]. Other presenting symptoms include headache, mental status changes, nausea, vomiting, vertigo, visual complaints and/ or focal weakness [2]. On diagnostic imaging, ODG’s appear as mass lesions with fairly well defined margins, usually located in the cortex and subcortical white matter [5]. Calcifications are common and are seen in 90% of ODG’s on CT scans [3]. On magnetic resonance spectroscopy (MRS), the lesion demonstrates an increase in choline (Cho) levels with elevated
choline/creatine ratio. Positron emission tomography (PET) scan has been reported to be able to differentiate between low grade astrocytoma’s and ODG’s [6]. Derlon et al. in his study found that both these tumor types exhibit glucose hypo-metabolism but on methionine (MET) PET imaging, the uptake of MET was high in the oligo-dendroglioma lesions and decreased, normal or only moderately increased in the astrocytoma group [6]. PET scan may also be useful for grading ODG’s non-invasively [7]. In a different study by Derlon et al. demonstrated that anaplastic ODG’s exhibited a higher 18F-fluro-deoxy-glucose and methionine (MET) uptake than low grade ODG’s [7].
On histological examination, the tumor cells contain uniformly round, homogenous nuclei and a clear cytoplasm. This typical “fried egg appearance” is actually an artifact of formalin fixation which is not present on frozen section and may make the diagnosis of ODG difficult on being frozen [8]. ODG’s may also display a dense network of branching capillaries which is described as “chicken wire” vascular pattern [8]. When the tumor cells invade the grey matter structures such as the cortex, these neoplastic oligodendrocytes tend to cluster around the neurons exhibiting a phenomenon known as “perineuronal satellitosis”. ODG’s usually do not stain for GFAP.
Features associated with anaplastic ODG’s (WHO grade III) and which differentiate these lesions from low grade ODG’s are contrast enhancement on CT or MRI; endothelial proliferation; pleomorphism; tumor proliferation (high mitotic count or high MIB-1 index) and astrocytic component. The high frequency of co-deletion of chromosomal arms 1p and 19q is a striking feature of this glial tumor and is considered as a “genetic signature” of ODG’s. 1p and 19q co-deletion has been associated with both chemosensitivity and improved prognosis in ODG’s [9]. The phosphatase and tensin homolog (PTEN) gene mutations on chromosome 10 are associated with a poor prognosis.
Surgery is the mainstay of treatment for ODG’s. The aim of surgery is gross total resection of the tumor, if possible. The extent of resection largely depends on the location of the tumor and its proximity to ‘eloquent’ areas of the brain. In most studies, the authors have concluded that more complete resection is associated with increased patient survival [10-12]. Post-surgery, radiation therapy is recommended for all patients with a diagnosis of anaplastic ODG regardless of the extent of resection. Radiation therapy is also recommended for patients of grade II ODG who have undergone an incomplete tumor removal or subtotal resection or biopsy of the lesion. Oligodendroglioma’s are chemosensitive tumors. Post-surgery, adjuvant chemotherapy with either PCV (procarbazine, CCNU or lomustine and vincristine) regimen or oral temozolomide is the standard of care [2]. PCV chemotherapy regimen was the most commonly used chemotherapy regimen to treat ODG’s but is now been superseded by oral temozolomide therapy. This is because of the ease of administration and fewer adverse effects with oral temozolomide therapy.
ODG’s, like all other infiltrating glioma’s have a high rate of recurrence and gradually increase in grade over time. Recurrent tumors are treated with re-surgery (if possible), more aggressive chemotherapy and radiation therapy. Median survival time for grade II ODG’s is 11.6 years and for anaplastic ODG’s is 3.5 years [13]. Factors associated with better prognosis in patients of ODG are age less than 40 years at the time of diagnosis; frontal lobe ODG; gross total resection of the tumor; lower grade of malignancy; good post-operative performance scores; early presentation of the tumor with seizures and combined deletion of chromosomal arms 1p and 19q. Pure ODG’s have a better prognosis than mixed oligoastrocytoma’s.
Conclusion
Corpus callosal oligodendroglioma is a rare entity with only a single case reported worldwide till date to our knowledge [1]. The treatment for anaplastic and aggressive ODG’s should include gross total resection (wherever possible) or tumor decompression followed by adjuvant concurrent chemo-radiation. This should be followed by oral temozolomide chemotherapy which in our case has given a good result at the end of 2 years post-surgery.
Contributors: CSG: manuscript writing, case management and literature search; SKS, DS: manuscript editing and intellectual inputs; RKS: histopathology and manuscript editing. CSG will act as guarantor. All authors approved the final version of the manuscript.
Funding: None; Competing interests: None stated.
References
- Monaco EA 3rd, Armah HB, Nikiforova MN, Hamilton RL, Engh JA. Grade II oligodendroglioma localized to the corpus callosum. Brain Tumor Pathol. 2011;28:305-309.
- Engelhard HH, Stelea A, Mundt A. Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment and prognosis. Surg Neurol. 2003;60:443-456.
- Mork SJ, Lindegaard KF, Halvorsen TB, Lehmann EH, Solgaard T, Hatlevoll R, et al. Oligodendroglioma: incidence and biological behavior in a defined population. J Neurosurg. 1985;63:881-889.
- Shah N, Pigott K, Bradford R. Intradural drop metastases in oligodendrogliomas. Clin Oncol. 1997;9:346-348.
- Lee YY, Van Tassel P. Intracranial oligodendrogliomas: imaging findings in 35 untreated cases. Am J Roentgenol. 1989;152:361-369.
- Derlon JM, Petit-Taboue MC, Chapon F, Beaudouin V, Noel MH, Creveuil C, et al. The in vivo metabolic pattern of low-grade brain gliomas: a positron emission tomographic study using 18F-fluorodeoxyglucose and 11C-L-methylmethionine. Neurosurgery. 1997;40:276-287.
- Derlon JM, Chapon F, Noel MH, Khouri S, Benali K, Petit-Taboue MC, et al. Non-invasive grading of oligodendrogliomas: correlation between in vivo metabolic pattern and histopathology. Eur J Nucl Med. 2000;27:778-787.
- Engelhard HH, Stelea A, Cochran EJ. Oligodendroglioma: pathology and molecular biology. Surg Neurol. 2002;58:111-117.
- Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636-645.
- Berger MS, Rostomily RC. Low grade gliomas: functional mapping resection strategies, extent of resection, and outcome. J Neurooncol. 1997;34:85-101.
- Shaw EG, Scheithauer BW, O’Fallon JR, Tazelaar HD, Davis DH. Oligodendrogliomas: the Mayo Clinic experience. J Neurosurg. 1992;76:428-434.
- Dehghani F, Schachenmayr W, Laun A, Korf HW. Prognostic implication of histopathological, immunohistochemical and clinical features of oligodendrogliomas: a study of 89 cases. Acta Neuropathol. 1998;95:493-504.
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479-489.