6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffa40b220000000f07000001000300
6go6ckt5b5idvals|855
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
The term Marjolin’s ulcer is used to describe the neoplastic transformation of a chronic ulcer or scar. Marjolin’s ulcer has been described from scars of all types and sinuses of chronic osteomyelitis [
1]. The first report of cancer arising in old burns scar has been attributed to Aureluis Cornelus Celsius in the 1st century AD [
2]. The tumor was however first described in depth by Jean-Nicholas Marjolin in 1828 and was defined as “ulcer-chanchroides” [
3] and by Dupuytren in 1839 [
4]. Professor Robert Smith in 1850 described the condition as the warty ulcer of Marjolin [
2]. However, it was Da-Costa in 1903 who coined the term Marjolin’s ulcer to describe malignant degeneration of skin scars especially in burns [
5]. The commonest type of malignant transformation is squamous cell carcinoma. Sarcomatous changes are rare, and within the sarcomas found in Marjolin’s ulcers, malignant peripheral neural sheath tumor is uncommon. We present a rare case of malignant peripheral neural sheath tumor occurring as a Marjolin’s ulcer.
Case Report
The patient is a 68 year old man, referred from an orthopedic hospital with a right gluteal ulcer. He sustained flame burns over the trunk and the right gluteal region when he was 13 years old. The burn wounds had healed without surgical intervention leaving a flat scar. There was no history of recurrent scar breakdown and healing of the scar. Three months prior to presentation, he was involved in road traffic accident and sustained a right femoral fracture. He was treated with skeletal traction for 12 weeks. During this period of admission, he developed right ischial decubitus ulcer on the burn scar in the right gluteal region.
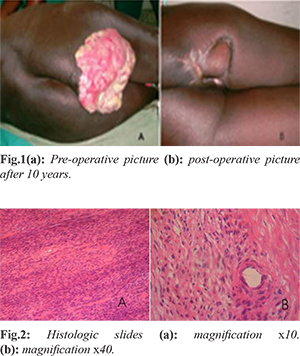
He was mobilized after fracture healing. The decubitus ulcer was treated with daily dressings. He declined surgical treatment and the ulcer continued to enlarge. He later developed contact bleeding associated with severe pain aggravated by walking. He presented back to the hospital and at presentation he was found to be hemodynamically unstable with hypotension and anemia. There was a right ischial ulcer measuring 20×20 cm with everted edges. There was also profuse, offensive discharge [Fig.1]. There was no inguinal lymph node enlargement, no family history or cutaneous stigmata of von Recklinghausen’s disease. Hematological investigations showed leucocytosis of 15,800/mm3, erythrocyte sedimentation rate of 125 mm/hr, and packed cell volume of 21%. Electrolytes, urea and creatinine along with electrocardiograph were normal. Fluid therapy and blood transfusion were instituted to stabilize patient and low molecular weight heparin was administered at 40 mg via a subcutaneous route daily as prophylaxis against deep venous thrombosis. A four quadrant incisional biopsy revealed a malignant peripheral neural sheath tumor.
An excision biopsy with 10 cm of unstretched skin was carried out; the excised specimen included the lower half of the gluteus maximus [Fig.1]. A tensor fascia lata myocutenous flap with de-epithelized pedicle was tunneled and used to reconstruct the defect. The histopathology revealed dense fibrocollagenous tissue occupied by a hyper-cellular mesenchymal lesion formed by elongated cells with thin fibrillar cytoplasm and spindle fairly uniform but hyper-chromatic wavy nuclei. There is variable cellularity with myxoid relatively pauci-cellular areas in which collagen is often disposed in a "shredded carrot appearance”. Mitoses are frequent in some areas. Immunohistochemistry showed that the tumor cells are negative for EMA, MNF116 and S100. The features are consistent with a malignant peripheral nerve sheath tumor. These results were consistent with the initial incisional biopsy report [Fig.2]. He was commenced on cyclical combination chemotherapy using cisplatin based regime. He received cisplatin 50 mg/m2 as a continuous infusion over 14 hours stat with intravenous adriamycin 50 mg/m2 as bolus. A total of 6 cycles was administered. Radiotherapy 60 Gy was also administered in fractions. He has remained free of tumor recurrence ten years after surgery [Fig.1].
Discussion
Marjolin’s ulcer is often seen in atrophic unstable post-burns scars, though it may also occur in chronic burn wounds. The following types of scars or ulcers have been found to transform into Marjolin’s ulcers; pressure sores, urinary fistulae, pilonidal sinuses, snake bite scars, acne condylomata, discoid lupus erythromatosis, osteomyelitis, hidradenitis suppurativa, scars of frost bite and skin graft donor sites and long term exposures to ultra-violet rays or ionizing irradiation [
2,
6].
The commonest histologic and most aggressive type is squamous cell carcinoma which accounts for 75-90% of all cases [
2,
6]. Other neoplastic histologic types include basal cell carcinoma, melanoma, and sarcomas [
2]. The following types of sarcomas were found, eight malignant fibrous histiocytoma, three fibrosarcoma, two liposarcoma, two dermato-fibrosarcoma protuberans, and there were one each of a malignant schwannoma, leiomyosarcoma, angiosarcoma, osteosarcoma, unspecified sarcoma, and mesenchymal tumor [
2]. In this same review of 412 tumors, one percent was found to be benign and did not fit into the definition of Marjolin’s ulcers [
2]. The benign histologic types found were keratoacanthoma, fibroxanthoma neurothelioma. In a study conducted by Melike Oruci et al. where 63 patients were studied for Marjolin’s ulcer over 15 years, no sarcoma was reported in the tumors. There were 88.9% squamous cell carcinoma and 11.1% basal cell carcinoma among the patients [
5].
The diagnostic criteria of Marjolin’s ulcer as postulated by Ewings require the presence of a burn scar and tumor that originates within the boundaries of the scar [
3]. There should also be no previous history of tumor in the location. The tumor histology must be compatible with cell types found in normal skin or scar tissues and there should be adequate interval between the injury and tumor development [
2]. The clinical case we report satisfies these criteria. Marjolin’s ulcers have a high propensity for metastasis and local recurrence. Early recognition and proper staging and treatment offers the best chance for cure [
7].
About 80% of all skin cancers are basal cell carcinoma, 16% squamous cell carcinoma and 4% malignant melanoma [
2]. In contrast, squamous cell carcinoma is the most frequent in burn scar neoplasm accounting for 71% of all skin cancers, basal cell type account for 12% and melanoma 6% [
2]. Overall, Marjolin ulcer accounts for about 2-3% of all skin cancers. In Nigeria however, the frequency is as high as 30% and unlike other parts of the world, the predisposing lesion has not been predominantly flame burns [
8]. The average age of presentation is 50 years, and the median latency period is from 20 to 35 years. They are typically found in the lower extremities 43.7%, upper extremity 22.4%, trunk 11.5%, head and neck 22.4%. Relapse rate is 58% and survival rate 52%, 34%, 33%, at 5 years, 10 years, and 20 years respectively [
8]. To best of our knowledge, this will be the second case of peripheral nerve sheath tumor reported from a Marjolin’s ulcer and the first from Africa. The first report was from a 57 year old woman who was managed by surgical excision alone on account of histologic margins. She subsequently developed local recurrences twice that necessitated re-excision. During the last recurrence she was found to have a pulmonary metastasis and received a course of epirubicin. The patient died 3 years after the initial diagnosis [
4]. In view of this finding in the literature, our patient was offered post-operative radiotherapy and chemotherapy inspite of the clear histologic margins at surgery. He has been free of recurrence after ten years.
Evidence has shown that the pathology of scar neoplasm differs from that of regular skin cancers. It is accepted that Marjolin’s ulcers arise following chronic irritation of scars with the scar tissue, providing an immunologically privileged site. Various hypotheses have been proposed regarding the pathogenesis of malignant transformation. These are inflammation and irritation from trauma precipitating malignant transformation. Exposure to chemicals may act as co-carcinogenic agents.
The absence of lymphatic channels and poor vascularity of scar tissue hinders the body defense mechanisms from having access to the tumor during oncogenesis. This last feature also is responsible for a delay in metastasis as in the patient presented with a locally advanced tumor with no lymph node or distant metastasis. Hereditary predisposition has been identified in the Human leukocyte Antigen DR4 system [
9].
Treatment modalities of this tumor are controversial. Surgical excision with wide margins of 5 cm offers potential for cure in early cases while adjuvant therapy is believed to be of limited value. It is recommended that prophylactic or therapeutic lymph dissection in addition to wide surgical margins be performed for tumors of the lower extremities due to the high rate of metastasis [
7,
10]. Others have advocated lymph node dissection based on histological grade [
10]. Eastman [
10] recommends that sentinel lymph node dissection should be the standard as in head and neck tumors as they provide a more accurate staging of the tumor. In the patient we reported, there was no lymph node enlargement and therapeutic lymph node dissection was not done.
Conclusion
Malignant neural sheath tumor is a very rare histological variant of burn scar tumor. Surgical excision of the primary tumor remains the gold standard for treatment. We recommend post-operative chemotherapy and radiotherapy despite negative tumor excision margin, as it appeared that it could be responsible for preventing recurrence inspite of the advance stage of the primary tumor. In the absence of lymph node involvement, prophylactic lymphadenectomy is not necessary.
Acknowledgement: Dr. UcheIgbokwe, Consultant pathologist, Department of Cellular Pathology, Oueen’s Hospital Romford, UK for his contribution to the histological diagnosis.
Contributors: OB: manuscript writing, literature search, patient management; BOM: manuscript editing, literature search, patient management; OOK, AOU: critical inputs into the manuscript and literature search. OB will act as guarantor. All authors approved the final version of this manuscript.
Funding: None; Competing interests: None stated.
References
- Phillips TJ, Salman SM. Burn scar carcinoma. Dermatol Surg. 1998;22:561-565.
- Kowal-Vern A, Criswell BK. Burn scar neoplasms. A literature review and statistical analysis. Burns. 2005;31:403-413.
- Ewing J. Neoplastic diseases. 3rd ed, Philadelphia 1928. pp.862.
- Scott JR, Morris R, McPhaden AR, Knight SL, Webster MH. Malignant schwannoma in a burn scar. Burns. 1996;22:494-496.
- Melike O, Kankaya Y, Veysel S. Clinicopathological evaluation of Marjolin ulcers over two decades. Kaohsiung J Medical Sciences. 2017;33:327-333.
- Ramsey ML. Basal cell carcinoma. In; Taylor RS, Butler DF, Quirk C, Elston DM. ed.E-Medicine: http//www.emedicine.com /DERM/topic47htm Accessed 5 June, 2018.
- Esther RJ, Lamps I, Schwartz HS. Marjolin ulcers: secondary carcinoma in chronic wounds. J South Orthop Assoc. 1999;8:181-87.
- Achebe JU., Akpuaka FC. Scar cancer in Nigeria: a retrospective study and review of literature. West Afr J Med. 1987;6:67-70.
- Aydog?du E, Yildirim S, Ako¨zT. Is surgery an effective and adequate treatment in advanced Marjolin ulcer? Burns. 2005;31:421-431.
- Eastman AL. Sentinel lymph node biopsy identifies occult nodal metastasis in patients with Marjolins ulcer. J Burn Care Rehabil. 2001;25:241-245.