6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffa4ed26000000de06000001000700
6go6ckt5b5idvals|913
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Hypertension has a worldwide prevalence of 30.8% and contributes markedly to death from stroke and coronary heart disease [
1]. Approximately 10% of all hypertensive patients are believed to have an underlying secondary cause, with an even greater prevalence below the age of 30 [
2]. The two commonest causes of secondary hypertension, namely primary aldosteronism (PA) and renovascular disease, are presented in this case series. In both conditions, patient may present with metabolic alkalosis and/or hypokalaemia.
Case Reports
Case 1: Primary Aldosteronism
A 27-year-old male of Filipino origin was referred to the renal team for consultation regarding hypertension. He had a strong family history of early onset hypertension on his paternal side. First diagnosed with hypertension at the age of 21, the patient was commenced on amlodipine 10 mg daily followed by addition of telmisartan 80 mg daily by his general practitioner. However, he was non-compliant with treatment, believing that these agents were not reducing his blood pressure. On presentation, his blood pressure was 170/100 mmHg with an otherwise unremarkable clinical examination.
Biochemistry showed normal renal function with serum potassium 3.3 mmol/L (3.2-5.0 mmol/L) and bicarbonate 30 mmol/L (22-32 mmol/L); demonstrating potassium at the lower and bicarbonate at the upper range of normal values respectively. Secondary causes of hypertension were evaluated in the context of early onset hypertension and strong family history. A plasma aldosterone concentration (PAC) of 547 pmol/L (32-654 pmol/L) and plasma renin concentration (PRC) of <2.0 mIU/L (2.8-39.9 mIU/L), with significantly raised PAC: PRC of >270 (normal <30) was suggestive of PA. Patient underwent sodium loading test and an unsuppressed serum PAC of 393 pmol/L (normal <277 pmol/L) post-intravenous saline loading was confirmatory for the diagnosis of PA. Subsequent computer tomography (CT) of his abdomen revealed that the medial limb of the left adrenal measured 15.69 mm in width (normal <3.3 mm) [3] suggesting unilateral adrenal hyperplasia [Fig.1]. Ideally, adrenal vein sampling (AVS) would be performed next to definitively identify lateralising aldosterone production, however the patient refused this test. Following diagnosis, he was commenced on spironolactone 25 mg daily in addition to his previous anti-hypertensives. His blood pressure is currently well controlled at 120/70 mmHg.
The patient’s 52-year-old father, who had a 25-year history of poorly controlled hypertension despite multiple anti-hypertensive agents, was also evaluated for PA. He had a PAC of 400 pmol/L (32-654 pmol/L), PRC of 2.8 mIU/L (2.8-39.9 mIU/L), and PAC: PRC of 143 (normal <30), again indicative of PA. CT abdomen revealed an 11 mm sized right adrenal lesion, though AVS was again refused. He was commenced on spironolactone 25 mg daily in addition to his usual medications but within one week the spironolactone dose needed to be halved due to symptomatic hypotension. Genetic testing for familial hyperaldosteronism (FH) type 1 (glucocorticoid remediable hypertension) was negative in both father and son. Absence of massive bilateral adrenal hypertrophy excluded FH type 3 and either FH type 2 or the rarer FH type 4 were therefore thought to be the most likely cause of hypertension in this family.
Case 2: Renal artery stenosis
A 64-year-old Caucasian lady was referred to the renal team for persistent hypokalaemia. She had a background of hypertension, hyper-cholesterolemia and left internal carotid artery angioplasty and stent. Her regular medications were perindopril 2.5 mg daily, amlodipine 10 mg daily, aspirin 100 mg daily and atorvastatin 20 mg daily. Her blood pressure on examination was 166/82 mmHg and apart from a right sided abdominal bruit, the remainder of the clinical examination was unremarkable.
Biochemistry showed normal renal function with serum bicarbonate of 33 mmol/L (22-32 mmol/L) and potassium of 2.0 mmol/L (3.2-5.0 mmol/L). Further investigations showed a raised PAC of 1000 pmol/L (32-654 pmol/L) and significantly raised PRC of 282.1 mIU/L (2.8-39.9 mIU/L) with a PAC: PRC of 4 (normal <30); values consistent with secondary aldosteronism. A CT angiogram of abdomen revealed near occlusion of the right renal artery at origin with small atrophic right kidney [Fig.2] suggesting renal artery stenosis (RAS). The patient’s perindopril dose was doubled to 5 mg daily whilst continuing the rest of her medications. Her blood pressure is currently well controlled at 120/80 mmHg.
Discussion
PA and reno-vascular disease are the most prevalent causes of secondary hypertension [
2]. Both PA and unilateral renovascular disease are characterised by raised plasma aldosterone concentration and may lead to metabolic alkalosis and/or hypokalaemia. However, the PRC is low in PA and raised in unilateral renovascular hypertension leading to a characteristically high PAC:PRC (>30:1) in the former. The various causes of secondary hypertension are outlined in
table 1. Patients with the following clinical clues should be screened for secondary hypertension [
2]:
• Severe or resistant hypertension (persistent hypertension despite = 3 antihypertensive medications from different classes at optimal doses, including a diuretic)
• Abrupt onset of hypertension or acute rise in blood pressure in a patient with previously stable values
• Onset of hypertension at <30 or >55 years of age
• Accelerated/malignant hypertension (severe hypertension with signs of end organ damage such as retinal haemorrhages or papilledema, heart failure, neurologic disturbance or acute kidney injury)
• Hypertension with metabolic alkalosis and/or hypokalaemia
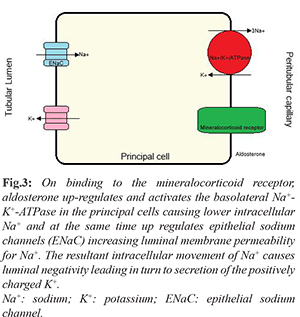
In the collecting duct, sodium is reabsorbed by luminal epithelial sodium channels (ENaC) into the principal cells. This sodium reabsorption is coupled with tubular secretion of potassium and mediated by aldosterone. However, as more positively charged sodium is reabsorbed than potassium gets excreted, positively charged hydrogen is additionally excreted by neighbouring alpha-intercalated cells into the tubule [
4,
5]. Aldosterone therefore causes tubular secretion of potassium and hydrogen with reabsorption of sodium
[Fig.3]. In non-epithelial tissues, excess aldosterone causes oxidative stress and collagen remodelling leading to endothelial dysfunction, left ventricular hypertrophy and fibrosis of heart, kidney and blood vessels [
6].
Up to 8% of the hypertensive population has PA, increasing to 20% prevalence in those with severe hypertension [
2]. Heart failure (3.7-fold increase), stroke (4.2-fold increase), myocardial infarct (6.5-fold increase) and atrial fibrillation (12.1-fold increase) can all develop as a consequence of excess aldosterone [
2]. The inappropriately high aldosterone in PA classically causes hypertension with metabolic alkalosis and hypokalaemia, though absolute hypokalaemiais seen in only 9-37% of cases [
7]. In addition to the patient groups recommended for secondary hypertension screening, detection of PA should be pursued in the following hypertensive patients [
6,
7]:
• Hypokalaemia provoked by administration of a low-dose diuretic
• Presence of adrenal incidentaloma
• Hypertensive first-degree relatives of patients with primary aldosteronism
• Family history of early onset hypertension or cerebrovascular accident at young age
PA screening tests consist of early morning serum PAC, PRC and a PAC:PRC ratio in a seated patient. An elevated PAC =15 ng/dL (416 pmol/l), a low PRC and PAC:PRA =30:1 and are highly suggestive of PA. Confirmatory tests aim to demonstrate lack of aldosterone suppression despite oral or intravenous sodium loading [
6]. An adrenal CT follows, aiming primarily to distinguish between unilateral and bilateral adrenal pathology [10]
[Table 2]. This is important as bilateral idiopathic hyperplasia (IHA) which causes 65% of PA is treated medically, while aldosterone producing adenomas (APA) or unilateral adrenal hyperplasia which make up 30% and 3% respectively of all cases of PA are treated surgically [
2]. However, CT scan alone has the following limitations in the diagnostic evaluation of PA [
6]:
• The prevalence of incidental adrenal tumour (incidentaloma > 1 cm) ranges from 1 to 9% and more than 80% of these are non-secretory and benign
• Bilateral lesions are not necessarily diagnostic of IHA, as patients with an APA may have a non-functioning benign mass in the contralateral adrenal
Adrenal vein sampling (AVS) is therefore used to definitively differentiate between lateralising or unilateral aldosterone production and bilateral disease [
7]. Laparoscopic unilateral complete adrenalectomy is the preferred management for unilateral excess aldosterone production, as seen in APA or unilateral adrenal hyperplasia. Up to 4 weeks post-operatively, the contralateral adrenal may continue to have suppressed aldosterone production and patients should be monitored for hyperkalaemia. In patients who have IHA or are not suitable for surgical intervention, a mineralocorticoid receptor antagonist such as aldosterone is the preferred treatment [
6,
7]. Familial hyperaldosteronism (FH) is an uncommon subset of primary aldosteronism and has four recognised types [
7,
8]:
• FH type I or glucocorticoid-remediable aldosteronism (GRA) is caused by a CYP11B1/CYP11B2 chimeric gene and associated with both cortisol and aldosterone production being controlled byplasma adrenocorticotrophic hormone (ACTH). Treatment consists of synthetic glucocorticoids to suppress ACTH secretion.
• FH type II has the largest prevalence amongst FH types, and is indistinguishable from sporadic primary aldosteronism occurring in more than one close family member. It is not associated with any identified mutation.
• FH type III is caused by germline mutations in the potassium channel subunit KCNJ5 and patients usually present early with massive bilateral adrenal hyperplasia which may occasionally need bilateral adrenalectomy
• FH type IV is caused by germline mutations in the CACNA1H gene which encodes a voltage-gated calcium channel. While CT scan may show APA, bilateral hyperplasia or normal appearing adrenal glands, AVS shows bilateral aldosterone hypersecretion.
Following PA, renovascular hypertension is the next most prevalent cause of secondary hypertension [
2]. More than 90% of renovascular hypertension is due to atherosclerosis causing RAS. Non-atherosclerotic fibromuscular dysplasia (FMD), which causes <10% of renovascular hypertension is mostly seen in women under the age of sixty years of age. In FMD, the stenotic lesion is typically more distal then in atherosclerotic RAS and usually has a beaded appearance [
9].
In unilateral RAS, hypoperfusion of the kidney leads to activation of the renin-angiotensin-aldosterone system (RAAS). The contralateral non-stenotic kidney compensates by increased excretion of sodium and water while the stenotic kidney remains hypoperfused. The RAAS remains chronically activated, leading to hyperaldosteronism and hypertension. In contrast, hypoperfusion of both kidneys in bilateral RAS impairs diuresis resulting in volume expansion [
10]. Thus, RAAS is suppressed in bilateral renovascular disease and the hypertension is due to volume expansion.
In addition to the usual recommendations for screening for secondary hypertension, diagnosis of RAS should be considered in hypertensive patients with [
9,
10]:
• Onset of severe hypertension (=180 mmHg systolic and/or 120 mmHg diastolic) after 55 years of age
• Acute rise in serum creatinine by >30% following the administration of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB)
• Unilateral small kidney or asymmetry in renal sizes >1.5 cm
• Recurrent flash pulmonary oedema without evidence of severely impaired left ventricular function
Due to lack of superiority of interventional procedures compared to medical management, investigation for RAS is only indicated if the detection of clinically significant renovascular disease would prompt a corrective procedure. Renal artery angiography remains gold standard for the diagnosis of RAS, however it is not routinely recommended because of the risks associated with this invasive procedure [
11]. Non-invasive screening tests include duplex Doppler ultrasonography, computed tomography angiography (CTA) and magnetic resonance angiography (MRA). Though Doppler ultrasound is relatively inexpensive, it is highly operator dependent and less diagnostic of the distal lesions typical in FMD. While MRA and CTA have the drawback of requiring contrast, they are both more sensitive than Doppler ultrasonography [
11]. A stenosis greater than 75% in one or both renal arteries is considered significant [
9]. Though not suitable for initial screening, captopril renogram may help to assess haemodynamic significance of the stenotic lesion and determine the relative function of each kidney [
12].
The first line management of atherosclerotic RAS is most often medical with the initial drug of choice in unilateral RAS being ACEI or ARB [10]. Bilateral renovascular disease, characterised by impaired sodium excretion and expanded plasma volume, benefits from addition of a diuretic to initiate natriuresis and overcome sodium retention in combination with ACEI or ARB [
13]. Serum creatinine should be monitored within 1-2 weeks of initiation of ACEI or ARB in these patients; a rise greater than 30% warrants discontinuation of these medications [
14].
Conclusion
The presence of metabolic alkalosis and/or hypokalemia in a hypertensive patient warrants screening for PA or renovascular disease. Identifying secondary causes of hypertension may lead to targeted anti-hypertensive therapy resulting in better management.
Acknowledgements: The authors of this case series would like to acknowledge the contributions of Dr Basim Alqutawneh (MBChB, FRANZCR, Master of medical ultrasound), staff radiologist at Blacktown Hospital, who assisted with preparation of the CT figures.
Contributors: JCJ: manuscript writing, patient management; RC: manuscript editing, patient management; ST: critical inputs into the manuscript, discussion, patient management. ST will act as guarantor. All authors approved the final version of this manuscript.
Funding: None; Competing interests: None stated.
References
- Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441-450.
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:e13-e115.
- Vincent JM, Morrison ID, Armstrong P, Reznek RH. The size of normal adrenal glands on computed tomography. Clin Radiol. 1994;49:453-455.
- Xanthakis V, Vasan RS. Aldosterone and the risk of hypertension. Curr Hypertens Rep. 2013;15:102-107.
- Wagner CA. Effect of mineralocorticoids on acid-base balance. Nephron Physiol. 2014;128:26-34.
- Mattson C, Young WFJ. Primary aldosteronism: diagnostic and treatment strategies. Nature Clinical Practitce Nephrology. 2006;2:198-208.
- Funder JW, Carey RM, Manetero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis and treatment: An endocrine society clinical practice guideline. JCEM. 2016;101:1889-1916.
- Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife. 2015;4:e06315.
- Wahab A, Alvi S. Renal artery stenosis - when to suspect and how to diagnose. JICC. 2011;1:40-43.
- Vahist A, Heller EN, Brown EJJ, Alhaddad IA. Renal artery stenosis: A cardiovascular perspective. American Heart Journal. 2002;143:559-564.
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Rosenfield KA, Creager MA, et al. ACC/AHA 2005 Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary. JACC. 2006;57(6):1239-1312.
- Sultana N, Begum SMF, Parveen R, Sarker AK, Mutsuddy P, Islam S, et al. DTPA Captopril Renogram: Still an invaluable tool for probability assessment in suspected cases of renovascular hypertension. Bangladesh J Nucl Med. 2015;18:131-134.
- Messerli FH, Bangalore S, Makani H, Rimoldi SF, Allermann Y, White CJ, et al. Flash pulmonary oedema and bilateral renal artery stenosis: the Pickering syndrome. European Heart Journal. 2011;32:2231-2237.
- Chrysochou C, Foley RN, Yong JF, Khavandi K, Cheng CM, Kalra PA. Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;17:1403-1409.