cefuroxim und alkoholcefuroxim nebenwirkungen cefuroxim fass cefuroxim heumann
From the Department of Dermatology, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh – 160012, India.
Corresponding Author:
Dr. Divya Seshadri
Email: divyaseshadriaiims@gmail.com
Abstract
Papuloerythroderma of Ofuji is an enigmatic entity. It is commonly idiopathic; at times, it may occur secondary to atopy, infections, malignancies or drugs. We report a patient who developed the characteristic generalized itchy eruption of confluent cobblestone-like papules, sparing the flexures, a few days after he was put on terbinafine, empirically, for suspected dermatophytosis. Other associated features included relative lymphopenia and dermatopathic lymphadenopathy. The rash responded to withdrawal of terbinafine and treatment with systemic corticosteroids. Terbinafine has a wide range of cutaneous adverse effects, but this is the first report of terbinafine-associated papuloerythroderma. We would like to bring to notice this important, though rare side effect, of a commonly used drug.
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff98aa01000000b500000001001200 6go6ckt5b5idvals|143 6go6ckt5b5idcol1|ID 6go6ckt5b5|2000F757Tab_Articles|Fulltext Terbinafine is widely used for dermatophytosis. The spectrum of cutaneous adverse effects of this drug has been expanding with time. Though generally well tolerated, it causes rashes in around 6% of recipients, requiring discontinuation in 1% [1]. Reported cutaneous side effects include urticaria, pityriasis rosea, erythema multiforme, erythroderma, SJS-TEN, subacute cutaneous lupus erythematosus, acute generalized exanthematous pustulosis, worsening of psoriasis, triggering of generalized pustular psoriasis, bullous pemphigoid, aggravation of SLE and facial pigmentation [1-7]. Till date, papuloerythroderma does not figure on this list.
Papuloerythroderma of Ofuji, an incompletely understood entity, is a generalized, itchy eruption of flat-topped, reddish confluent papules, cut through by unaffected skin folds [8]. At times, it can be drug-related. We report, for the first time, a papuloerythroderma-like drug rash secondary to terbinafine.
Case report
A 68 year old man, presented with a two-year history of thickening, discolouration and transverse grooving of all nails and a one-year history of recurrent itchy vesicular lesions on the hands and feet. Scrapings and nail clippings showed no fungal elements, but he was empirically started on terbinafine 250 mg/day. He was not on any other medication at that time. Four days after starting terbinafine, he began to develop itchy erythematous papules on the legs. Over the next week, the eruption became generalised. Examination revealed confluent, erythematous, infiltrated, firm, cobblestone-like papules involving around 80% of the body surface ( figure 1). The intervening background skin was mildly erythematous. The face and truncal folds were relatively spared; the cubital and popliteal fossae were completely spared (figure 2). The patient was afebrile; vital signs and systemic examination were normal. Few soft cervical lymph nodes, smaller than 1 cm were palpable.
Blood work revealed a haemoglobin of 13 g/dL, platelet count of 2 lakhs/µL, leucocytosis with a total count of 21000/µL, relative lymphopenia with 74% neutrophils, 20% lymphocytes, 4% monocytes and 2% eosinophils and a normal peripheral blood film. Liver and kidney function tests were normal. Skin biopsy showed epidermal acanthosis, spongiosis, occasional necrotic keratinocytes and a dense upper dermal perivascular lymphohistiocytic infiltrate with few neutrophils. Serum IgE levels were not obtained. Prostate examination, urine and stool routine examination, and chest and abdomen CTs were within normal limits. Terbinafine was stopped and the patient was put on oral corticosteroids. After ten days of prednisolone 60 mg/day, most lesions had completely resolved. While on treatment, the dystrophic nails were shed and normal nails began to grow in their place. The palm and sole vesicles responded to topical steroids.
A papuloerythroderma-like eruption secondary to terbinafine was diagnosed. It was also concluded that his original complaints represented dyshidrotic eczema with secondary nail dystrophy.
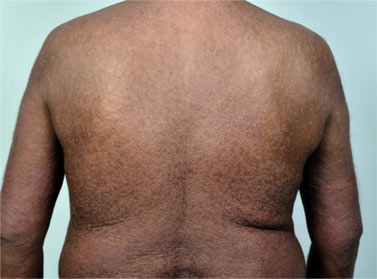 Figure 1 – Coalescing, cobblestone-like reddish-brown papules on the trunk
Figure 1 – Coalescing, cobblestone-like reddish-brown papules on the trunk
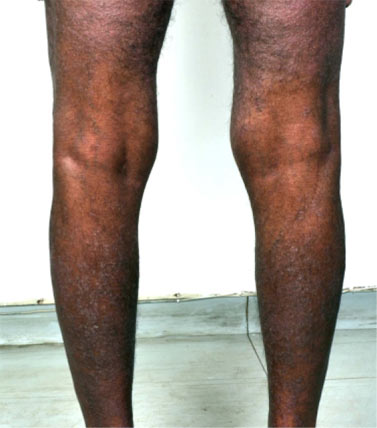 Figure 2 – Sparing of the popliteal fossae
Discussion
Papuloerythroderma, first described by Ofuji et al [8], is an enigma of sorts. Debate continues regarding whether it is a distinct clinical entity or merely a manifestation pattern of several underlying diseases [9,10]. A recent review concluded that most cases are idiopathic but some are associated with atopy, malignancies, including cutaneous lymphomas and internal malignancies, infections and drugs [11]. An isolated report associates systemic nickel allergy [12]. A recent series suggests that co-existent internal malignancy may be commoner than expected [13]. Among the reported cases, drug-induced papuloerythroderma accounts for less than 5% [11]. So far, the implicated drugs include nifedipine, dideoxyinosine, isoniazid, aspirin, furosemide, ranitidine, eperisone hydrochloride, leuprorelin acetate, ticlopidine and imatinib [10,14]. Reported drug-to-rash intervals range from 2 weeks to 10 years; longer latencies have been associated with intermittent administration of the culprit drug. Drug provocation tests and lymphocyte transformation tests, when done, have been found positive, but patch tests have been uniformly negative. Drug-specific lymphocytes are polarised towards a Th-2 phenotype, which may explain the lack of demonstrable contact sensitivity [14].
The hallmark of Papuloerythroderma of Ofuji (PEO) is a generalized, itchy eruption of flat-topped, red-brown coalescing papules, cut through by unaffected skin folds and creases (deck-chair sign). Palmoplantar keratoderma is described in one-fifth and dermatopathic lymphadenopathy in a fourth of patients [11]. It almost exclusively affects elderly males. Histopathology is usually non-specific or compatible with chronic dermatitis, except in those cases that have turned out to be CTCL. Other associated findings include eosinophilia, raised serum IgE and lymphopenia. Successful treatment modalities include systemic corticosteroids, cyclosporine, azathioprine, PUVA, UVB, interferon a and systemic retinoids [11,15].
Recently, papuloerythroderma has been classified as primary PEO (idiopathic), secondary papuloerythroderma, i.e., linked to atopy, malignancy, infections or drugs, papuloerythroderma-like CTCL and pseudopapuloerythroderma [11]. Diagnostic criteria have been proposed for the primary and secondary varieties. Necessary criteria include the characteristic eruption, deck-chair sign, itch and histopathological exclusion of other diseases. Primary PEO further requires work up to exclude links with atopy, malignancies, infections or drugs. Minor criteria include age above 55, male gender, peripheral and/or tissue eosinophilia, increased serum IgE and peripheral lymphopenia [11]. Our patient fulfilled all necessary criteria for secondary papuloerythroderma and three out of five minor criteria. The strong temporal relationship of his eruption to terbinafine was noteworthy and points to its role as a trigger. To our knowledge, papuloerythroderma induced by terbinafine has not been reported earlier.
Conclusion
The growing list of drugs causing papuloerythroderma-like eruptions necessitates greater awareness and recognition of this entity. With regard to terbinafine, we need to consider its role when a patient on this drug develops a widespread papular eruption sparing the folds.
References
Figure 2 – Sparing of the popliteal fossae
Discussion
Papuloerythroderma, first described by Ofuji et al [8], is an enigma of sorts. Debate continues regarding whether it is a distinct clinical entity or merely a manifestation pattern of several underlying diseases [9,10]. A recent review concluded that most cases are idiopathic but some are associated with atopy, malignancies, including cutaneous lymphomas and internal malignancies, infections and drugs [11]. An isolated report associates systemic nickel allergy [12]. A recent series suggests that co-existent internal malignancy may be commoner than expected [13]. Among the reported cases, drug-induced papuloerythroderma accounts for less than 5% [11]. So far, the implicated drugs include nifedipine, dideoxyinosine, isoniazid, aspirin, furosemide, ranitidine, eperisone hydrochloride, leuprorelin acetate, ticlopidine and imatinib [10,14]. Reported drug-to-rash intervals range from 2 weeks to 10 years; longer latencies have been associated with intermittent administration of the culprit drug. Drug provocation tests and lymphocyte transformation tests, when done, have been found positive, but patch tests have been uniformly negative. Drug-specific lymphocytes are polarised towards a Th-2 phenotype, which may explain the lack of demonstrable contact sensitivity [14].
The hallmark of Papuloerythroderma of Ofuji (PEO) is a generalized, itchy eruption of flat-topped, red-brown coalescing papules, cut through by unaffected skin folds and creases (deck-chair sign). Palmoplantar keratoderma is described in one-fifth and dermatopathic lymphadenopathy in a fourth of patients [11]. It almost exclusively affects elderly males. Histopathology is usually non-specific or compatible with chronic dermatitis, except in those cases that have turned out to be CTCL. Other associated findings include eosinophilia, raised serum IgE and lymphopenia. Successful treatment modalities include systemic corticosteroids, cyclosporine, azathioprine, PUVA, UVB, interferon a and systemic retinoids [11,15].
Recently, papuloerythroderma has been classified as primary PEO (idiopathic), secondary papuloerythroderma, i.e., linked to atopy, malignancy, infections or drugs, papuloerythroderma-like CTCL and pseudopapuloerythroderma [11]. Diagnostic criteria have been proposed for the primary and secondary varieties. Necessary criteria include the characteristic eruption, deck-chair sign, itch and histopathological exclusion of other diseases. Primary PEO further requires work up to exclude links with atopy, malignancies, infections or drugs. Minor criteria include age above 55, male gender, peripheral and/or tissue eosinophilia, increased serum IgE and peripheral lymphopenia [11]. Our patient fulfilled all necessary criteria for secondary papuloerythroderma and three out of five minor criteria. The strong temporal relationship of his eruption to terbinafine was noteworthy and points to its role as a trigger. To our knowledge, papuloerythroderma induced by terbinafine has not been reported earlier.
Conclusion
The growing list of drugs causing papuloerythroderma-like eruptions necessitates greater awareness and recognition of this entity. With regard to terbinafine, we need to consider its role when a patient on this drug develops a widespread papular eruption sparing the folds.
References
- Gupta AK, Lynde CW, Lauzon GJ, Mehlmauer MA, Braddock SW, Miller CA, et al. Cutaneous adverse effects associated with terbinafine therapy: 10 case reports and a review of the literature. Br J Dermatol 1998;138:529–532.
- Bangsgaard N, Saunte DM, Folkenberg M, Zachariae C. Serious adverse events reporting on systemic terbinafine: a Danish register-based study. Acta Derm Venereol 2011;91:358–359.
- Kasperkiewicz M, Anemüller W, Angelova-Fischer I, Rose C, Zillikens D, Fischer TW. Subacute cutaneous lupus erythematosus associated with terbinafine. Clin Exp Dermatol 2009;34:e403–404.
- Ozturk G, Turk BG, Karaca N, Karaarslan IK, Ertekin B, Ertam I, et al. Generalized pustular eruptions due to terbinafine. Cutan Ocul Toxicol 2012;31:81–84.
- Aksakal BA, Ozsoy E, Arnavut O, Ali Gürer M. Oral terbinafine-induced bullous pemphigoid. Ann Pharmacother 2003;37:1625–1627.
- Terrab Z, El Ouazzani T, Zouhair K, El Kabli H, Lakhdar H. Terbinafine-induced Stevens-Johnson syndrome and aggravation of systemic lupus erythematosus. Ann Dermatol Venereol. 2006;133:463–466.
- Breuer K, Völker B, Gutzmer R, Kapp A, Werfel T. Facial pigmentation following therapy with terbinafine. Hautarzt 2005;56:1056–1059.
- Ofuji S, Furukawa F, Miyachi Y, Ohno S. Papuloerythroderma. Dermatologica 1984;169:125–130.
- Ofuji S. Papuloerythroderma. J Am Acad Dermatol 1990;22:697.
- Bettoli V, Pizzigoni S, Borghi A, Virgili A. Ofuji papulo-erythroderma: a reappraisal of the deck-chair sign. Dermatology 2004;209:1–4.
- Torchia D, Miteva M, Hu S, Cohen C, Romanelli P. Papuloerythroderma 2009: two new cases and systematic review of the worldwide literature 25 years after its identification by Ofuji et al. Dermatology 2010;220:311–320.
- Kato K, Satoh T, Tanaka T, Ueda N, Yokozeki H. Systemic nickel allergy presenting as papuloerythroderma-like eruptions. Acta Derm Venereol. 2010;90:655–656.
- Teraki Y, Aso Y, Sato Y. High Incidence of Internal Malignancy in Papuloerythroderma of Ofuji: A Case Series from Japan. Dermatology 2012;224:5-9.
- Sugita K, Kabashima K, Nakamura M, Tokura Y. Drug-induced papuloerythroderma: analysis of T-cell populations and a literature review. Acta Derm Venereol 2009;89:618–622.
- Bech-Thomsen N, Thomsen K. Ofuji’s papuloerythroderma: a study of 17 cases. Clin Exp Dermatol 1998;23:79–83.
|