|
Gianfranco Lapietra1, Francesca Fazio1, Federico Vozella1, Francesca Maria Pirolli2, Maria Teresa Petrucci1 1Department of Hematology, Department of Translational and Precision Medicine, Sapienza University, Rome, Italy; 2Department of Emergency Radiology, Sapienza University, Rome, Italy.
Corresponding Author:
Dr Maria Teresa Petrucci Email: petrucci@bce.uniroma1.it
Abstract
Background: Multiple myeloma (MM) is a common hematologic malignancy. Immunomodulants, inhibitors of proteasome and monoclonal antibodies represent the standard of care regimens. Neurologic complications could occur in MM patients, both related to the disease or to the therapy. Posterior reversible encephalopathy syndrome (PRES) is a rare disease occurring with a several neurologic symptoms (seizures, headaches, visual loss and confusions) and characterized by typical imaging. It has been described in patients affected by malignancies but only few cases have been reported in MM patients on treatment. Case Report: We herein report the case of a 71 year-old woman with relapsed-refractory micro-molecular MM on treatment with immunomodulants who developed a clonic-tonic seizure during hospitalization for acute kidney failure. The brain computed-tomography (CT) scan and the magnetic resonance imaging (MRI), performed after the event, showed pathologic variations of cortical and subcortical signal of posterior cerebral regions. These findings were considered to be consistent with PRES. The cerebral lesions gradually disappeared, due to supportive therapy. The patient died to hematologic disease progression few months later. Conclusion: The pathophysiology of PRES is still unclear. Further studies are warranted to better understand its relation with multiple myeloma on treatment.
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffe8d62f0000006e07000001000800 6go6ckt5b5idvals|2022 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Multiple myeloma (MM) is a plasma cells neoplasm that accounts for 10% of hematologic malignancies [ 1]. In the last years, survival rates have improved significantly due to introduction of new drugs, such as immunomodulants, proteasome inhibitors and monoclonal antibodies that represent the standard of care regimens. The quality of life and the life expectancy of myeloma patients may be worsened by various complications, both related to the disease or to therapy: infections, bone disease, hematologic problems, venous thromboembolism and neuropathies. The most frequent form of neurotoxicity in myeloma patients is the peripheral neuropathy, seen in up to 20% of patients at diagnosis and it can be experienced by up to 75% of patients during therapy [ 2]. The posterior reversible encephalopathy syndrome (PRES) is a neurologic central disorder of rapid onset that usually occurs with seizures, visual loss, confusions and headaches. The typical findings at magnetic resonance imaging (MRI) are white matter vasogenic edema affecting the posterior occipital and parietal cerebral lobes. Epidemiologic data are missing due to the diagnostic difficulties but today it is increasingly recognized because of improvement of brain imaging [ 3]. The pathophysiology of this syndrome is unclear. Even if it usually occurs in patients with hypertension, it has been described in different conditions, including hematologic and non-hematologic malignancies. To best of our knowledge, only four cases of PRES in multiple myeloma patients have been reported. In this subset of patients, PRES could be a form of neurotoxicity, due to disease itself or to therapy. We herein report the case of a patient with a relapsed-refractory micro-molecular multiple myeloma developing PRES during tenth line of therapy with immunomodulants.
Case Report
A 71-year old woman was admitted to the Accident and Emergency Department of our hospital with acute kidney failure. The patient had been diagnosed with multiple micro-molecular myeloma six years earlier. She received chemotherapy induction according to VTD scheme (bortezomib, thalidomide and dexamethasone) followed by autologous hematopoietic stem cell transplant. She relapsed after one year and underwent nine more lines of therapy, including proteasome inhibitors, monoclonal antibodies and immunomodulators. At the time of admittance, she was being treated with immunomodulators. On arrival, the patient was asymptomatic with mild anemia, an increase in creatinine level (4 mg/dL) and hyponatremia (125 mg/dL). Bence-Jones proteinuria was significantly increased compared to the levels of her last outpatient visit. A nephrologic evaluation was performed and she was hydrated to adjust the electrolytic imbalances. Few days later, she developed a mild increase of blood pressure which required specific therapy and we decided to keep her in hospital. One week later, the patient had a generalized tonic-clonic seizure that was refractory to diazepam and lorazepam and she was given a continuous infusion of midazolam. A brain computed-tomography (CT) scan was performed. It showed hypodensities of cortical and subcortical white matter in the posterior regions of both cerebellar hemispheres and in the occipital lobes, extending into the frontal and parietal regions bilaterally. The patient then underwent a magnetic resonance imaging (MRI). Fluid-attenuated inversion recovery (FLAIR), T2 sequences and diffusion weighted imaging (DWI) demonstrated multiple foci of hyperintensity in both hemispheres in supratentorial and infratentorial regions [Fig.1]. These radiologic aspects were considered to be consistent with posterior reversible encephalopathy syndrome (PRES). The patient was transferred to the neurologic department where she gradually recovered. During her hospital stay, she developed a Herpes genitalis infection which required therapy with acyclovir. The MRI performed ten days after the neurologic event, showed an almost complete disappearance of the previous described pathological lesions. The patient was treated with valproic acid and then discharged. She was restarted immunomodulatory therapy but a further disease evaluation showed a disease progression and she was given a palliative therapy. The patient died few months later.
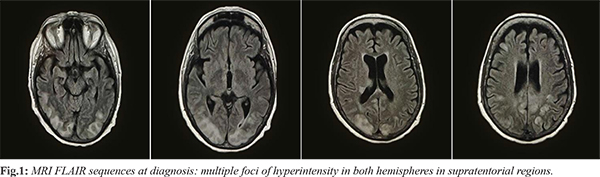
Discussion
PRES is a neurologic disorder which can occur with a variety of symptoms, for example headaches, seizures, confusion and visual loss being the most common [ 3]. The pathophysiology of this disease is still unclear. It is mainly observed in patients with hypertensive crisis: the leading hypothesis is that it is connected to vascular leakage and edema is caused by cerebral hyper-perfusion during a rapid increase in blood pressure [ 4]. The paucity of sympathetic innervation seems to be the cause of an increased susceptibility of the posterior areas of the cerebral hemispheres to vasomotor dysregulation. PRES has also been observed in patients with normal blood pressure, affected by infectious diseases, kidney diseases or hematologic and solid malignancies, on treatment with cytotoxic chemotherapy or with immunosuppressants [ 5- 8]. All these conditions are characterized by presence of endogenic or exogenic toxins which can damage the cerebral endothelium, inducing vascular oedema. Several cases of chemotherapy-related PRES in patients affected by hematologic malignancies have been reported in literature [ 8], but PRES in patients treated for multiple myeloma has been previously described in only four cases. Two patients, both treated with immunomodulants developed seizures and painless vision loss, respectively [ 9, 10], and one patient developed headaches, cortical blindness, ocular paresis, and hypertension, during DT-PACE salvage chemotherapy scheme (dexamethasone, thalidomide, cisplatin, adriamycin, cyclophosphamide, and etoposide) [ 11]. Garfall et al. described PRES after infusion of anti-BCMA CAR-T cells, possibly due to high levels of cytokines in the cerebrospinal fluid, during cytokines-release syndrome [ 12]. The neurological symptoms in all these cases were accompanied by typical signs of neuroimaging. Neurotoxicity in multiple myeloma can be related to the disease (compression, infiltration, metabolic and immune alterations), or to the drugs, especially in the form of peripheral neuropathy, while little is known about central neuropathy. For instance, some studies have shown that immunomodulants cross the blood-brain barrier in murine models and in non-human primates [ 13, 14] and one of their targets seems to be a gene involved in the pathogenesis of mental retardation [ 15]. Our patient’s neurological complication did not appear to be related to hypertension as the increase in blood pressure was mild. It did not seem to be related to herpetic infection, either which was localized and well controlled, nor with the multiple myeloma, as the neuroimaging did not show any nervous localization of the disease. It could have been related with the renal impairment, even though, at the time of the neurologic complication, kidney function was improving.
Conclusion
The reported case remains one of the few cases of PRES observed in a patient with active MM. In patients with active MM, PRES could be a form of neurotoxicity, due to disease itself or to therapy. Further studies are warranted to better understand its mechanism and its link with plasma cell neoplasms on treatment.
Contributors: GL, FF: manuscript writing, literature review; FV, MTP: manuscript editing, references. FMP: critical inputs into the manuscript and imaging. MTP will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of the study. Funding: None; Competing interests: None stated.
References - Rajkumar, SV. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018;93:1091-1110.
- Richardson PG, Delforge M, Beksac M, Wen P, Jongen JL, Sezer O, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595-608.
- Sudulagunta SR, Sodalagunta MB, Kumbhat M, Nataraju AS. Posterior reversible encephalopathy syndrome (PRES). Oxf Med Case Reports. 2017; 2017(4):omx011.
- Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914-925.
- Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006; 27:2179-2190.
- Vaughn C, Zhang L, Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr Oncol Rep. 2008;10:86-91.
- Ganesh K, Nair RR, Kurian G, Mathew A, Sreedharan S, et al. Posterior reversible encephalopathy syndrome in kidney disease. Kidney Int Rep. 2018;3:502-507.
- Connolly RM, Doherty CP, Beedy P, et al. Chemotherapy induced reversible posterior leukoencephalopathy syndrome. Lung Cancer. 2007;56:459-463
- Chow S, Cheung CS, Lee DH, Howson-Jan K, Xenocostas A. Posterior reversible encephalopathy syndrome in a patient with multiple myeloma treated with thalidomide. Leuk Lymphoma. 2012;53:1003-1005.
- Pandey R, Patel A, Shah S, Patel KM, Shah PM, Shukla SN. A rare complication in a case of multiple myeloma on therapy with thalidomide and dexamethasone-Reversible posterior lobe leukoencephalopathy. Leuk Lymphoma. 2006;47:1431-1434.
- Tam CS, Galanos J, Seymour JF, Pitman AG, Stark RJ, Prince HM. Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies. Am J Hematol. 2004;77:72-76.
- Garfall AL, Lancaster E, Stadtmauer EA, Lacey SF, Dengel K, Ambrose DE, et al. Posterior reversible encephalopathy syndrome (PRES) after infusion of anti-BCMA CAR T cells (CART-BCMA) for multiple myeloma: successful treatment with cyclophosphamide. Blood. 2016;128:5702.
- Muscal JA, Sun Y, Nuchtern JG, Dauser RC, McGuffey LH, Gibson BW, et al. Plasma and cerebrospinal fluid pharmacokinetics of thalidomide and lenalidomide in nonhuman primates. Cancer Chemother Pharmacol. 2012;69:943-947.
- Li Z, Qiu Y, Personett D, Huang P, Edenfield B, Katz J, et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS One. 2013;8:e71754.
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345-1350.
|