6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff24ea310000007a06000001000200
6go6ckt5b5idvals|2051
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Cough, fever, and shortness of breath are hallmark symptoms of COVID-19; however, an increasing number of cases report major cardiac complications related to the viral infection [1]. Most reports have focused on cardiovascular events associated with acute cardiac injury, defined by cardiac biomarkers above the 99th percentile upper reference limit [2]. We describe three patient presentations of distinct arrhythmias without evidence of respiratory compromise or elevated cardiac biomarkers in Covid-19 infected patients in Lawrence, Massachusetts. The three patients described in the case series were treated between the end of March and the end of May, 2020, during a period when Lawrence experienced a significant surge of COVID-19 patients in a primarily LatinX patient population.
Case Series
Case 1
A 78-year-old male with a history of chronic kidney disease, hypertension, diet-controlled type II diabetes, benign prostatic hypertrophy, and depression presented to the Emergency Department (ED) with a ten-day history of weakness, anorexia, and cough, as well as several syncopal episodes at home. The patient reported several days of dizziness upon standing, but denied any chest pain, dyspnea, or palpitations. He was unsure if he ever lost consciousness or had any seizure-like activity and denied any history of syncope, gait instability, or frequent falls preceding this acute illness.
Vital signs at presentation: temperature of 100.8 F, pulse 81 beats/min, blood pressure 151/81 mm Hg, respiratory rate 16/min, pulse oximetry 96% on room air. Orthostatic vital signs obtained after receiving a 1 L normal saline bolus were not consistent with hypovolemia. On examination, the patient was alert and oriented with an unremarkable exam aside from crackles in the bases of both lungs. The EKG was unremarkable. Chest X-ray demonstrated no interval focal airspace disease. Pertinent laboratory values during hospitalization are noted in Table 1. His troponin was negative and the rest of his labs were unremarkable. Patient was placed on cardiac telemetry. Advanced imaging was not obtained due to institutional protocols related to his pending SARS-CoV-2 RT-PCR.
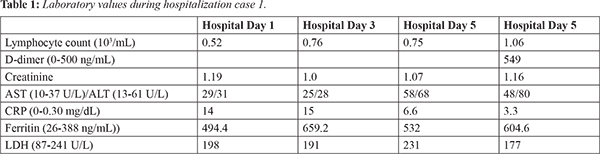
On hospitalization day 2 at 12:15 pm the patient had an episode of dizziness and emesis, during which his rhythm changed from normal sinus to complete heart block followed by 12 seconds of asystole and subsequently returned to normal sinus rhythm. Transcutaneous pacers were put on the patient and atropine was placed at bedside. Approximately 1 hour later, the patient had two recurrent episodes of complete AV block followed by asystole lasting 7 and 14 seconds, then converted back to normal sinus rhythm. Cardiology was consulted, but possible interventions were limited due to his pending COVID-19 status. Infectious disease was consulted and the patient was started on hydroxychloroquine that evening. He received one dose of 400 mg that was discontinued the next day due to concern for arrhythmogenic effects. On hospitalization day 3, the patient developed an oxygen requirement of 4L/per minute, but remained in normal sinus rhythm. On hospitalization day 4, his Sars-CoV-2 PCR returned positive. The patient reported a pre-syncopal episode while lying in bed, which correlated with a 20 second period of complete AV block without asystole on telemetry. During the subsequent hospitalization, the patient was weaned off of oxygen, and his strength and appetite improved. He was monitored on telemetry with no recurrence of AV block or asystole, and daily EKGs were unremarkable. The patient was discharged on hospitalization day 8 with close cardiology follow-up and orders for a 30-day heart monitor and echocardiogram. Our cardiology consult planned for further outpatient work-up, including possible pacemaker placement, if these episodes were recurrent.
Case 2
A 79-year-old male with COPD, chronic mesenteric ischemia, type II diabetes, hypertension and known aortic stenosis was admitted after presenting to his primary care clinic in new onset atrial fibrillation. His chief complaint was a four-day history of progressive exertional dyspnea associated with headache and abdominal pain. Notably, he denied any cough and fever. The patient had a significant cardiac history. His most recent transthoracic echocardiogram in 2016 showed moderate to severe aortic stenosis, ejection fraction (EF) of 55%, impaired LV relaxation, and mild concentric LV hypertrophy. Additionally, an exercise stress test in 2016 was notable for non-sustained atrial fibrillation while in recovery, but negative for ischemia. In February 2017, he was hospitalized for dizziness and chest pain and was noted to have a non-sustained asymptomatic run of atrial fibrillation that lasted 40 seconds. He was discharged with a 48-hour Holter monitor that revealed frequent premature atrial contractions (PACs) and premature ventricular contractions and a five-beat run of paroxysmal supraventricular tachycardia (PSVT).
Vital signs at presentation: temperature 98.4 F, pulse 104 beats/min, blood pressure 101/85 mmHg, respiratory rate 20/min, and pulse oximetry 98% on room air. His labs, including a troponin series, were negative. An EKG showed persistent atrial fibrillation with rates in the 90-110s. A portable chest x-ray was read as early viral pneumonia vs atelectasis in the left lung base. Patient was started on a diltiazem drip and converted to normal sinus rhythm that evening.
On hospitalization day 2, he was transitioned to 180 mg PO diltiazem daily and had no additional episodes of atrial fibrillation during his stay. He was found to be COVID-19 positive on this day. His pertinent labs are summarized in Table 2, which shows the evolution of the inflammatory markers commonly seen in COVID-19 infections. He did not develop respiratory distress or an oxygen requirement during his stay. He was started on Eliquis 5 mg BID. The patient’s symptoms resolved, and his blood pressure and heart rate were well controlled at his follow-up.
Case 3
A 67-year-old male with history of obesity, type II diabetes, hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED two days after a single syncopal episode associated with urination at night. Following the episode, he returned to bed as was asymptomatic. Two days later, he called his primary care office and was advised to go to the ED. Vital signs at presentation: temperature 98.6 F, pulse 136 beats/min, blood pressure 132/91 mmHg, respiratory rate 18/min, pulse oximetry 100% on room air.
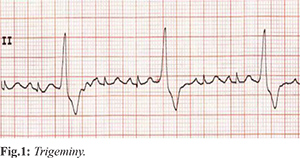
EKG showed tachycardia and ventricular trigeminy [Fig.1]. A portable chest x-ray noted poor definition of the left heart border thought most likely to be pericardial fat pad, and CTA chest revealed peripheral ground glass opacities posteriorly in the right upper lobe. Labs were notable for a WBC 4.3×103/µL and lymphocyte count of 1.12×103/µL. Troponins were negative. His tachycardia improved after a 2L bolus of normal saline. He was admitted to telemetry for monitoring. On hospitalization day 2 his SARS-CoV-19 PCR resulted positive. He continued to deny symptoms, but remained tachycardic with rates 100-120s and repeat EKGs demonstrated frequent unifocal PVC’s. On hospitalization day 3, his temperature rose to 101.6 F. He never developed respiratory symptoms or an oxygen requirement. His creatinine increased during his stay to a peak of 1.40 mg/dL on hospital day 4 (day of discharge) and outpatient labs demonstrated a return to baseline (1.10 mg/dL) the following week. Prior to discharge his tamsulosin was changed to finasteride. Two days after discharge EKG showed sinus tachycardia without PVCs. In visits during the following weeks, he continued to deny respiratory symptoms or shortness of breath.
Discussion
COVID-19 is defined by severe respiratory illness, an increasing number of studies report acute cardiac abnormalities in patients with the virus [3]. This is consistent with previous viral epidemics including the 2002 coronavirus outbreak and annual influenza outbreaks, for which complications include myocarditis, acute myocardial infarction, cardiomyopathy and heart failure exacerbation [4-6]. A recent single center cohort study reported 19.7% of hospitalized patients with COVID-19 experienced cardiac injury, defined as blood levels of cardiac biomarkers above 99th percentile upper reference limit, which was associated with an unexpected high risk of mortality during their stay [3]. Other studies have noted acute cardiac injury as a complication associated with mechanical ventilation and poor prognosis [4].
The three cases described here are unique because the patients did not require mechanical ventilation and did not demonstrate elevated troponin levels signaling myocardial injury. These patients presented only with cardiac symptoms instead of more common symptoms such as cough and fever; the first and the third patients experienced syncope while the second patient noted palpitations and exertional dyspnea. It has been noted that acute myocarditis and ventricular arrhythmias may represent the first clinical manifestation of SARS-CoV-2 infection for some patients and it is postulated that arrhythmia may be the cause of sudden cardiac death in non-hospitalized patients with mild symptoms who were found dead at home while in isolation [7].
There are limited studies describing arrhythmias induced by novel COVID-19, and even fewer describing isolated presentations of arrhythmia in COVID-19 patients. A recent report of 138 patients hospitalized with COVID-19 found that 16.7% of patients were found to have arrhythmias, with a higher incidence among patients in the ICU [4]. Another study of 187 patients noted that patients with elevated troponin T (TnT) levels more frequently developed complications including malignant ventricular arrhythmias (11.5% vs 5.2%). Interestingly, it was also reported that mortality was significantly higher in patients with elevated TnT levels (59.6% vs 8.9%) [2].
The understanding of how COVID-19 affects the heart is limited at this time; however, a number of models have been proposed. One of the ways that SARS-CoV is proposed to cause direct myocardial injury is through binding to angiotensin-converting enzyme (ACE 2) which is present in lung, heart, kidney, and GI tissue. It is postulated that this is the primary receptor through which viral entry occurs, allowing the virus to subsequently alter ACE2 pathways, leading to acute myocardial injury [8]. Of note, digestive organs, kidneys, and the heart contain significantly more ACE2 than pulmonary tissue, which may explain frequent presentations of GI symptoms and renal injury in hospitalized patients with COVID-19 [9]. A consideration regarding myocardial injury is that a patient may develop atrial or ventricular fibrosis, predisposing them to develop cardiac arrhythmias. Further monitoring of patients who have had evidence of myocardial injury due to COVID-19 will be important to better understand and stratify the arrhythmic risk of those infected [1].
Additional mechanisms of injury are thought to be caused by systemic inflammation and increased myocardial oxygen demand, leading to acute cardiac injury and increasing risk of acute coronary syndromes, heart failure, and arrhythmias. The hypoxemia caused by COVID-19 may also trigger atrial fibrillation, the most common arrhythmia among elderly individuals, especially those with underlying structural heart disease [10]. It is important to note that therapies proposed as treatments for COVID-19 have known effects on myocyte repolarization and the capacity to potentiate arrhythmias, thus their use should be evaluated carefully in patients with cardiac symptoms.
Each of the patients described in this series were noted to have arrhythmias originating from different locations along the electro-cardiac conduction pathway. All of the patients were in their 70s and carried a diagnosis of essential hypertension, characteristics previously noted to be associated with coronavirus-induced cardiac injury [3]. What is most notable about these cases is that none of the patients presented with respiratory symptoms or demonstrated acute cardiac injury indicated by elevated cardiac markers. Additionally, two of the three cases had no history of underlying structural cardiac disease. These atypical presentations of COVID-19 infection show a significant risk for missed diagnosis in institutions that are not universally testing individuals.
Conclusion
Finally, these cases highlight the need for further investigation into the cardiovascular effects of Sars-CoV2, with or without evidence of acute cardiac injury to aid in the development of concrete epidemiological evidence.
Contributors: ZLG: Manuscript writing, literature review and patient management; JAS, literature review, manuscript writing, patient management, and manuscript editing; KS, PON: manuscript writing and patient management; RJ: manuscript editing and patient management. JAS will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of the study.
Funding: None; Competing interests: None stated.
References
- Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in Covid-19 patients. J Cardiovasc Electrophysiol. 2020;31(5):1003-1008.
- Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5(7):811-818.
- Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
- Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1(3):274-281.
- Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798-1800.
- Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):819-824.
- De Wit E, Van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nature Reviews Microbiology. 2016;8:523.
- Cheng P, Zhu H, Witteles RM, Wu JC, Quertermous T, Wu SM, et al. Cardiovascular risks in patients with COVID-19: Potential mechanisms and areas of uncertainty. Current Cardiology Reports. 2020;22(5):34.
- Yang C, Jin Z. An acute respiratory infection runs into the most common noncommunicable epidemic-COVID-19 and cardiovascular diseases. JAMA Cardiol. 2020;5(7):743-744.