6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffd44003000000ea01000001000500
6go6ckt5b5idvals|208
6go6ckt5b5idcol1|ID
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Nucleoside analogue reverse transcriptase inhibitors (NRTI) have been used to treat HIV-infected patients for more than 20 years, and although their range of toxicity is considered mild, some cases of severe and even fatal toxicity have been described mainly attributed to mitochondrial dysfunction. First case of lactic acidosis associated with stavudine was described in 1997 [
1]. NRTIs are associated with several adverse events such as peripheral neuropathy, myopathy, pancreatitis, lactic acidosis, lipodystrophy and hepatic steatosis [
2]. We report a unique case of stavudine induced hepatic steatosis, hyperlactatemia, lipodystrophy, peripheral neuropathy, dyslipidemia and hyperglycemia in a patient along with zidovudine induced anemia.
Case Report
A 34 year old married female patientwas referred to Department of Gasteroenterology and Hepatology for abnormal liver function tests (LFTs) and hepatomegaly in June 2012. She was diagnosed with HIV infection in 2001 during routine medical examination. There was history of blood transfusion in 1999 during caesarean section. The patient was asymptomatic until 2006, when she presented with oral candidiasis. In March 2007, her CD4 count was 129 (Normal 800-1200). She was started on lamivudine, stavudine and nevirapine combination therapy. After two months of therapy, patient was symptomatic in the form of nausea, occasional vomiting, fatigue and abdominal pain in right hypochondrium. Her LFTs were mildly deranged. Ultrasonography of abdomen showed hepatomegaly (16 cm). Over a period of one year she had persistent abnormal LFTs and progressive enlargement of liver. In March 2008, she noticed peripheral fat loss with central fat accumulation. Her CT-abdomen showed fatty liver with hepatomegaly (21cm). Due to persistent abnormal LFTs and hepatomegaly, her regimen was changed to zidovudine, lamivudine and nevirapine in August 2008. In November 2008, she was transfused blood for anemia (Hb- 5.6 gm%) and she was advised to avoid both zidovudine and stavudine. Due to financial constraints, she was not started on protease inhibitors or alternative therapy. Inspite of physician advice she continued with stavudine, lamivudine and nevirapine for another year till November 2009. She did not follow up with her physician throughout 2011. Since April 2012, she had painful burning sensation in both feet with occasional numbness. She was referred to our department with above complaints and was admitted for further investigations.
During hospital stay, she was diagnosed with diabetes mellitus and dyslipidemia. In view of adverse effects she was shifted to a combination therapy of tenofovir, lamivudine and nevirapine. She was also started on oral hypoglycaemic drugs. On examination her vitals were stable. Her abdomen examination was suggestive of hepatomegaly of 12 cm below right costal margin which was soft, non-tender with round margin, with no ascites and splenomegaly.
Appropriate investigations were performed to rule out possible causes of liver transaminase elevations. The patient was negative for hepatitis B surface antigen (HBsAg), plasma HBV-DNA, hepatitis C virus antibodies (HCV-Ab) and HCV-RNA. Her antinuclear antibody and anti-smooth muscle antibody were negative and serum electrophoresis was not suggestive of hypergammaglobulinemia. Her cholesterol was 203 mg% (Normal range 50-250), triglyceride-484 mg% (Normal less than 170), random blood sugar-234 mg/dl (Normal range 80-120), Lactate-21.30 mg/dl (Normal range 4.5-16). Liver biopsy showed hepatocytes having marked macrovesicularsteatosis (80-90%), with glycogenated nuclei and no cholestasis [Fig.1]. Her CD4 count in June 2012 was 654. Her CT abdomen in June 2012, revealed hepatomegaly with fatty liver.
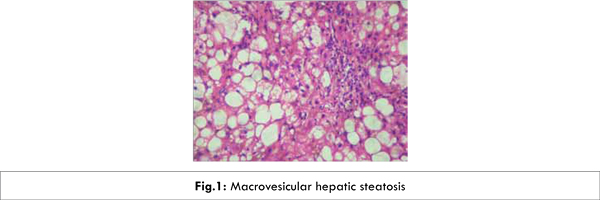
Patient was diagnosed to have stavudine induced hepatic steatosis, hyperlactatemia, lipodystrophy, neuropathy, dyslipidemia and hyperglycemia along with zidovudine induced anemia. Followup in December 2012, showed normal LFTs [Table 1]. Clinically the patient was asymptomatic.
Discussion
NRTIs are associated with several adverse events such as peripheral neuropathy, myopathy, pancreatitis, nephrotoxicity, lactic acidosis, and hepatic steatosis. Etiology has not been clearly established to date, but diverse mechanisms are proposed including inhibition of enzymes such as mitochondrial DNA gamma-polymerase (an enzyme that contributes to DNA replication), which causes decrease in energy generation, increase in lactate, toxicity, and cell death [
3]. Interference with mitochondrial metabolism also account for fat deposit in liver because fatty acids are oxidised at this site. Hepatic steatosis in a patient receiving NRTI may be an early sign of mitochondrial dysfunction [
4]. The link between NRTI therapy and mitochondrial damage was first suggested in 1990. Combination of NRTI has been shown to have additive or synergistic effect on mitochondrial dysfunction in vitro [
5]. NRTI associated lactic acidosis is almost always associated with hepatic steatosis. It is now recognized that by interfering with lactate production or its clearance in an organ or tissue, NRTIs can produce a spectrum of hyperlactatemia syndromes, including asymptomatic hyperlactemia or symptomatic hyperlactemia which may or may not be associated with hepatic steatosis [
6].
Lactic acidosis is a well known adverse effect caused by antiretroviral therapy. In our patient, there was asymptomatic hyperlactatemia. The natural history of asymptomatic hypertactatemia is not well defined. It is uncertain whether individuals with asymptomatic hyperlactatemia are at increased risk of developing symptomatic hyperlactatemia [
6]. A subset of HIV-1 infected patients undergoing antiretroviral treatment develops a lipodystrophy syndrome. It is characterized by loss of peripheral subcutaneous adipose tissue (face, limbs, buttocks), visceral fat accumulation, and in some cases, lipomatosis, especially in the dorso-cervical area [
7].
The major toxicities of NRTI therapy, particularly over the medium-term to long-term, include myopathy (zidovudine); neuropathy (stavudine, didanosine, zalcitabine); hepatic steatosis and lactic acidaemia (didanosine, stavudine, zidovudine); and possibly also peripheral lipoatrophy (possibly all NRTIs, although predominantly with stavudine); and pancreatitis (didanosine) [
8].
The uniqueness of our case is the presence of multiple side effects in a single patient comprising of hepatic steatosis, peripheral neuropathy, lipodystrophy, diabetes, dyslipidemia and hyperlactatemia. Stavudine induced lipoatrophy has been reported with low-grade lactic acidaemia and liver dysfunction, but in the absence of lipid or glycaemic changes [
9]. Contrary to this case, in our case we found association with dyslipidemia and hyperglycemia. Hyperglycemia and dyslipidemia are adverse effects of protease inhibitors and less commonly associated with NRTIs [
2,
9]. The anemia seen in our patient was probably related to zidovudine therapy.
After reviewing the literature; to the best of our knowledge our case is unique because no other case appears to be reported with multiple side effects in a HIV infected patient. All these adverse effects in this patient could be due to prolonged therapy (four years) because of irregular follow up. The patient could not be shifted to alternative therapy due to financial constraint and continued with same therapy.
Conclusion
This case highlights the potential toxicity of NRTIs in particular stavudine due to inadvertent prolonged use, which can be prevented by alternative therapy, regular follow up and early detection.
References
- Lenzo N, Garas B, French B. Hepatic steatosis and lactic acidosis associated with stavudine treatment in a HIV patient. A case report. AIDS. 1997;11:1294-1296.
- Daniel M. Lugassy, Brenna M. Farmer, Lewis S. Nelson. Metabolic and hepatobiliary side effects of antiretroviral therapy (ART). Emerg Med Clin N Am. 2010;28:409–419.
- Tanaka K, Fukahori S, Jojima H, Fujimatsu Y, Shiraishi K, Tanaka M, et al. Fatal lactic acidosis in a patient with acquired immunodeficiency syndrome treated with stavudine, lamivudine and indinavir.Kansenshogaku Zasshi. 1999;73:1232-1235.
- Chariot P, Drogou I, de Laroix-Szmania I, Elizer-Vanerot MC, Chazud B, Lombes A, et al. Zidovudine induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis and mitochondrial DNA depletion. J Hepatol. 1999;30:156-160.
- Menezes CN, Maskew M, Sanne I, Crowther NJ, Raal FJ. A longitudinal study of stavudine-associated toxicities in a large cohort of South African HIV infected subjects. BMC Infect Dis. 2011;11:244.
- Smith KY. Selected metabolic and morphologic complications associated with highly active antiretroviral therapy. J infect Dis. 2002;185:s123-s127.
- Villarroya F, Domingo P, Giralt M. Drug-induced lipotoxicity: lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim Biophys Acta. 2010;1801:392-399.
- Andrew Carr, David A Cooper. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430.
- Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14:F25–32.