6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffa84803000000ea01000001000900
6go6ckt5b5idvals|210
6go6ckt5b5idcol1|ID
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Small intestine constitutes 70% of the bowel. Cancer of the small bowel is rare, accounting for 0.3% to 1% of gastro-intestinal malignancies [
1]. In a 1972 autopsy study, the incidence was 0.019% to 0.5% [
2]. Nonetheless, the reported incidence has been increasing owing to the widespread use of endoscopy and good imaging techniques. Adenocarcinomas are the most common of small bowel malignancies, followed by carcinoid tumours, lymphomas, and leiomyosarcomas. Most of these small bowel adenocarcinomas are located in the duodenum, making this the most favoured site [
3]. We report a case of a 55-year-old female suffering from this small bowel malignant tumour.
Case Report
A 55-year-old female, a non-smoker, presented to our hospital with history of epigastric fullness, anorexia, repeated episodes of vomiting, malena and weight loss. There was no history of haematemesis and recent change in bowel habits. Physical examination demonstrated succussion splash and no other significant finding were noted. Baseline blood investigations showed normal liver function tests. With this history gastric outlet obstruction was suspected.
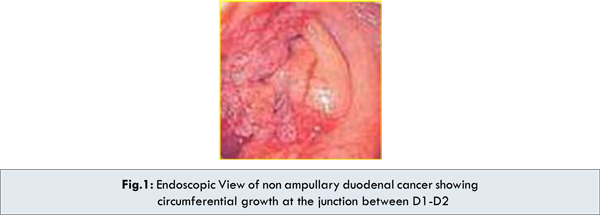
An oesophago-gastro-duodenoscopy was performed revealing circumferential narrowing of the beginning of 2nd part of duodenum and scope could not be negotiated beyond the point in view of swelling of the papilla and surrounding duodenal mucosa [Fig.1]. Endoscopic biopsy obtained twice to rule out malignancy revealed inflammation & dysplasia.
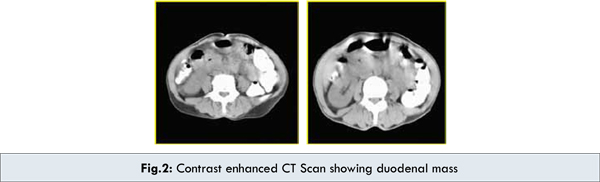
Computed tomography revealed a circumferential wall thickening involving 2nd part of duodenum with significant luminal narrowing [Fig.2]. Few lymph nodes were noted in para-aortic & aorto-caval regions. Strong possibility of malignancy or infective pathology especially tuberculosis was kept. CT guided FNAC yielded diagnosis of adenocarcinoma of duodenum. Patient was explained the condition and the patient agreed to undergo laparotomy.
A 3-4 cms stenotic mass was noted at D1 - D2 junction, with obvious metastasis in the transverse mesocolon near right colic vessel. Whipple’s operation was performed, and involved portion of transverse mesocolon along with segment of transverse colon was resected [Fig.3]. Gross pathological examination of the surgical specimen found a 4-cm focally indurated region just distal to the ampulla of Vater. On microscopy a well differentiated adenocarcinoma with columnar cells extending through the muscularispropria was demonstrated [Fig.4]. The common bile duct, pancreas, stomach, and peritoneum were not affected. 2 out of the 13 sampled lymph nodes were involved.
The diagnosis of adenocarcinoma, pT3N1M0 (American Joint Committee on Cancer [AJCC] classification), of the duodenum was established. His postoperative course was uneventful. The patient was asymptomatic at 6-months follow-up.
Discussion
Though small intestine constitutes about 70% of bowel,tumors of the small intestine are remarkably rare and constitute 0.3 to 1%. In duodenum, ampullary and periampullary carcinomas are common, making nonampullary carcinoma still rare [
4]. Rarity of the disease is because rapid transit time of liquid content of food, an alkaline environment, presence of benzopyrene hydroxylase, lower bacterial population and presence of IgA.
Most cases are sporadic, associations with familial adenomatous polyposis (FAP), Crohn’s disease [
5], Peutz-Jeghers syndrome [
6], and neurofibromatosis [
7] have been reported. With early diagnosis and treatment of colonic polyposis, adenocarcinoma of the duodenum has become the leading cause of death in FAP patients. A 10-year prospective study of 114 FAP patients revealed that six died of adenocarcinoma of the duodenum [
8].
The presentation of this disease is vague and non-specific. Patients can present with abdominal pain, bleeding, weight loss, obstruction, or jaundice. It should therefore be considered a differential diagnosis in patients presenting with epigastric discomfort. First-line investigations remain oesophagogastroduodenoscopy and contrast studies, which can usually demonstrate the site, severity, and length of the lesion. While more proximal tumours can be picked up by oesophagogastroduodenoscopy, more distal lesions by radiology but what is more important is a clinical suspicion, computed tomography is required in all biopsy-confirmed cases for staging and planning of treatment.
A diagnostic dilemma sets in when mucosal biopsy taken during upper endoscopy shows inflammation, as in our case. This can represent tuberculosis which is common in our country especially when there are enlarged lymph nodes or a complication of peptic ulcer disease or changes caused by reaction to a tumour in the vicinity, including carcinoma of the duodenum, pancreas, bile duct; or other lesions like gastro-intestinal stromal tumours or lymphomas. Endoscopic ultrasound may help by measuring the thickness of the lesion and allowing deeper biopsy of the lesion. Alternatively, especially when the obstruction does not allow the scope to pass, computed tomography and PET can help. The demonstration of a mass lesion necessitates an operation. An intra-operative frozen section may be required to reach a final diagnosis and guide the surgery.
Surgery remains the only potential cure for this type of tumour. Radical pancreaticoduodenectomy (Whipple’s operation) is the classical curative operation and is still the treatment of choice for tumours in the first and second parts of the duodenum. Some authors advocate duodenal segmentectomy as curative treatment for lesions in the third and fourth parts [
4]. Tocchi et al described duodenal segmentectomy with intestinal derotation, allowing a macroscopically clear margin with adequate lymphadenectomy [
4].
The mortality and morbidity rates were lower than those after Whipple’s operations. There is growing evidence that there is no difference in survival rates after Whipple’s operations and duodenal segmentectomies. There are also sporadic case reports describing successful endoscopic resections of early tumours that have not invaded the submucosa (Tis and T1) [
9].
In cases where the tumour has advanced beyond the possibility of curative resection, bypass surgery and stenting have a palliative role. Radiotherapy is not applicable as the tumour is radio-resistant and the small bowel has poor tolerance to radiation. Chemotherapy has no role in primary treatment and there is only limited information about its use as anadjuvant treatment. It is mainly reserved for recurrent disease.
The 5-year survival rate for curatively resected adenocarcinomas of the duodenum is of the order of 50 to 60%. This is better than those for other lesions in the vicinity, namely tumours of the ampulla, distal bile duct, and head of pancreas [
10]. Nodal involvement and the possibility of curative resection are independent prognostic factors for adenocarcinomas of the duodenum. In one prospective study, all the patients with N0 disease given curative surgery survived at 5 years, while only 47% of those with N1 disease were still alive at 5 years [
10]. Node-positive patients also have a statistically higher recurrence rate than their node negative counterparts [
11]. None of the patients with unresectable disease survived for 5 years.
Conclusion
Adenocarcinoma of the duodenum remains a rare disease and Whipple’s procedure remains to be main stay for treatment of non ampullary duodenal carcinoma.
References
- Gore RM. Small bowel cancer. Clinical and pathologicfeatures. Radiol Clin North Am. 1997;35:351-360.
- Cortese AF, Cornell GN. Carcinoma of the duodenum. Cancer 1972;29:1010-1015.
- Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors and outcome of 217 patients. Cancer 2004;101:518-526.
- Tocchi A, Mazzoni G, Puma F. Adenocarcinoma ofthe third and fourth portions of the duodenum: results of surgical treatment. Arch Surg. 2003;138:80-85.
- Mansari OE, Parc Y, Lamy P, Parc R, Tiret E, Beaugerie L. Adenocarcinoma complicating Crohn’s disease of theduodenum. Eur J Gastroenterol Hepatol. 2001;13:1259-1260.
- Nakamura T, Suzuki S, Yokoi Y. Duodenal cancer in apatient with Peutz-Jeghers syndrome: molecular analysis. J Gastroenterol. 2002;37:376-380.
- Joo YE, Kim HS, Choi SK, Rew JS, Park CS, Kim SJ. Primary duodenal adenocarcinoma associated with neurofibromatosis type 1. J Gastroenterol. 2002;37:215-219.
- Groves CJ, Saunders BP, Spigelman AD, Philips RK. Duodenal cancers in patients with familial adenomatouspolyposis (FAP): results of a 10-year prospective study. Gut. 2002;50:636-641.
- Friedrich-Rust M, Ell C. Early stage small bowel adenocarcinoma: a review of local endoscopic therapy.Endoscopy. 2005;37:755-759.
- Sarela AI, Brennan MF, Karpeh MS, Klimstra D, Conlon KC. Adenocarcinoma of the duodenum: importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann Surg Oncol. 2004;11:380-386.
- Barnes G Jr, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73-78.