6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff587305000000b802000001000100
6go6ckt5b5idvals|296
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Acute transverse myelitis (ATM) is a relatively uncommon neurological disorder characterized by inflammation of the spinal cord [
1]. The inflammatory process often extends longitudinally over three or more segments and functionally transects the entire substance of the cord. This condition has diverse etiologies and has a well-known association with vaccinations especially hepatitis B vaccine [
1]. Besides, several bacterial and viral infections are potential triggers for acute transverse myelitis. Acute transverse myelitis following hepatitis B virus infection has not been described in the literature. This case highlights the first report of acute transverse myelitis being a possible extra-hepatic manifestation of acute hepatitis B infection. The clinical presentation of transverse myelitis in this case had several overlaps with those of syringomyelia. In this report we discuss the significance of this new association of transverse myelitis and hepatitis B infection as well as some of the pitfalls in the clinical diagnosis of this condition.
Case Report
A 24 year old male smoker presented with sudden onset of weakness and loss of sensation over both upper limbs for 2 days. The weakness was symmetric, involving distal and proximal muscles and had not progressed since its onset. A few hours after onset of limb weakness, he noticed urinary retention as well. He also developed constipation a day later. There was no history suggestive of cranial nerve deficits, autonomic dysfunction or diffuse cerebral and brainstem involvement. The lower limbs were conspicuously spared. There was neither root pain nor localized vertebral pain. Low grade fever was present for 2 days, associated with malaise and anorexia.
On examination, he was conscious and co-operative with a pulse rate of 88/min and blood pressure of 110/70 mm Hg. General physical examination was within normal limits. Optic fundi showed no evidence of optic neuritis. Neurologic examination revealed normal bulk and tone of muscles. Proximal and distal muscle groups of both upper limbs demonstrated power of grade 2/5. Deep tendon reflexes were absent in the upper limbs. The patient was unable to sense pain and temperature along the distribution of C5 to T1 segments of the cord. However, fine touch, vibration and position sense were preserved in these areas, producing a characteristic dissociated sensory loss. Examination of lower limbs was normal, except for bilateral extensor plantar responses. In view of urinary retention, patient was on continuous bladder drainage.
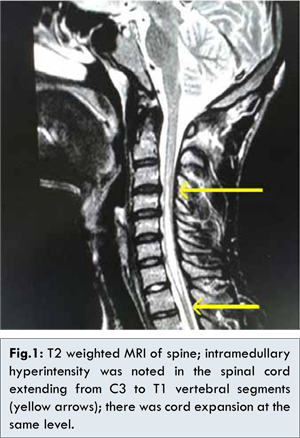
The major differential diagnoses considered were syringomyelia, acute transverse myelitis and multiple sclerosis. Acute onset was against the diagnosis of syrinx, whereas involvement of more than three spinal segments and normal white matter on MRI brain made multiple sclerosis unlikely. Magnetic Resonance Imaging (MRI) of the spine [Fig.1] demonstrated intramedullary T2 hyperintensity with central hypointensity in the cervical spinal cord extending from C3 to T1 level with cord expansion, without any contrast enhancement, suggestive of acute transverse myelitis. Cerebrospinal fluid (CSF) analysis showed mild pleocytosis of 8 lymphocytes/µL with normal protein and glucose levels.
Workup for the underlying cause was carried out, including serology for human immunodeficiency virus (HIV). Hepatitis B surface antigen (HBsAg) was positive and further evaluation provided evidence for acute HBV infection in the form of IgM antibodies to the Hepatitis B core antigen (IgM anti-HBcAg).Liver functions and ultrasonogram of abdomen were normal. Autoimmune workup including anti-nuclear antibodies (ANA) was negative.
Following a neurology and neurosurgical consult, we started him on pulses of methyl prednisolone injection followed by oral prednisolone. After five days of steroid, minimal neurologic improvement was noted in the form of return of pain sensation. By the end of second week, we were able to remove the urinary catheter and by the third week, there was return to normal power in both upper limbs. Steroid was tapered over six weeks and stopped.
Discussion
Acute transverse myelitis is characterized by protean neurologic deficits that develop over a period of several hours and progress over the next few days to weeks [
2]. Spinal cord dysfunction is usually complete and involves the entire cross section of the cord, producing the typical transverse myelopathy. There is motor weakness of the limbs and trunk depending on the segments involved and sensory disturbances mostly in the form of reduced or absent sensations with a definite upper level. Cervical and thoracic cords are commonly involved [
3]. In most cases, sooner or later, sphincter disturbances also appear [
2]. Frank autonomic failure is less common.
Syringomyelia on the other hand develops insidiously with a typical suspended segmental sensory loss, often leading to a cape like distribution of sensory deficit involving the upper extremities [
4]. There is often distal muscle weakness and wasting, and painless burns of fingers. Sparing of the posterior columns and segmental encroachment of the spinothalamic tracts by the expanding syrinx leads to the phenomenon of dissociated sensory loss. In our case, the patient had an acute presentation of symmetric weakness of upper limbs and dissociated sensory loss of upper limbs. This led us to the initial clinical diagnosis of syringomyelia of cervical cord with an acute onset. Such presentations of syrinx have been reported to occur following spinal trauma [
5]. However, there was no antecedent trauma in our patient. Other etiologies for syringomyelia include spinal tumors, congenital anomalies and myelomalacia. Association of syringomyelia with inflammatory disorders of nervous system is extremely rare.
MRI of the spine has become an invaluable tool in the diagnosis of acute transverse myelitis. In our case, the diagnostic dilemma was between structural abnormalities versus inflammation of the cord. MRI revealed intramedullary T2 hyperintensity extending from C3 to T1 segments. Thus our case fulfilled the diagnostic criteria by the Transverse Myelitis Consortium Working Group [
6], which consists of bilateral signs or symptoms of spinal cord dysfunction, a definite sensory level, spinal cord inflammation as evidenced by CSF pleocytosis or MRI findings and progression to peak neurologic deficits between 4 hours and 21 days.
The imaging in this case is typical of a longitudinally extensive transverse myelitis (LETM), a term that has become widely accepted following several recent studies [
7,
8]. This term is used to distinguish non-compressive spinal cord dysfunction in which MRI demonstrates T2 hyperintensity of the gray matter extending over 3 or more vertebral segments [
9]. Although etiologically LETM encompasses several groups of diseases like infections, inflammation, radiation, autoimmunity and malignancy, its significance probably lies in its contrast to the myelitis associated with multiple sclerosis. Studies have demonstrated that when acute transverse myelitis occurs as part or harbinger of multiple sclerosis, fewer than three segments show imaging abnormality [
10]. Moreover, cord expansion as was seen in our case, is also more common in LETM without any identifiable causes [
11].
In a review of vaccine related acute transverse myelitis [
1], hepatitis B vaccine was found to be the most common vaccine associated with LETM. Several others like Oral Polio vaccine (OPV), diphtheria-tetanus-pertussis (DTP) and influenza vaccines have also been reported to cause this adverse event [
1]. Apart from vaccines, a host of infectious agents have been implicated to cause transverse myelitis. The list includes mostly viruses such as Ebstein Barr virus, HIV, HTLV-1, Hepatitis A and Hepatitis C virus and bacteria like Mycoplasma pneumonia and Mycobacterium tuberculosis [
12,
13]. Parasites like Schistosoma and Ascaris have also been reported as possible etiological agents [
13]. However, Hepatitis B infection has not been reported to trigger acute transverse myelitis. This is quite interesting as vaccine against the same virus is among the most frequent cause for transverse myelitis. In the case of post-vaccinal and post-infectious transverse myelitis, many mechanisms have been proposed including molecular mimicry [
14], polyclonal B cell activation and epitope spreading [
15]. The end result of these phenomena is development of autoimmunity. It is logical therefore, to hypothesize that this was the case in our patient as well.
Since ATM is a rare disorder, there are no well-established management guidelines. The treatment in most cases is immunosuppression, steroids being the first line agents [
13]. The scenario however, is complicated when an infective association is identified. Our patient had an acute hepatitis B infection, and therefore the opinion on steroids was divided. Considering the degree of functional disability and after discussion with the patient, we arrived at the consensus to start steroids with careful monitoring of the liver functions and viral load. Following initiation of steroid, neurologic deficits improved slowly but steadily without any evidence of worsening liver functions or heightened virologic activity. Other therapeutic options available are plasma exchange, azathioprine, methotrexate and Infliximab [
13]. Intravenous immunoglobulin has no role in the management of ATM [
13].
Our case highlights certain important issues related to ATM. First, clinical diagnosis of ATM can be challenging especially if lower limbs are spared and atypical features as in our case are present. A combination of clinical features, CSF findings and MRI abnormalities (especially suggestive of LETM) should be used to achieve an early diagnosis. A short segment transverse myelitis and normal MRI of the brain significantly reduces the likelihood of multiple sclerosis related ATM. Second, apart from a search for autoimmune disorders, a broad infectious workup is essential to identify a post-infectious etiology. This should include serology for hepatitis B as well. Third, early treatment with steroids may alter the outcome of this devastating disease, and hence this option should be exercised judiciously. It might be beneficial even in the setting of an acute infection, if adequately monitored, as evidenced by our case.
Conclusion
Longitudinally extensive transverse myelitis is a distinct clinico-radiologic entity that is being increasingly recognized. The Transverse Myelitis Consortium Working Group criterion enables more objective diagnosis of ATM especially when clinical features are atypical. Although rare and not reported to date in literature, screening for hepatitis B infection should be part of workup of any case of ATM. Early and judicious treatment with steroids may have a remarkable effect on the outcome of the disease.
References
- Agmon-Levin N, Kivity S, Szyper-Kravitz M, Shoenfeld Y. Transverse myelitis and vaccines: a multi-analysis. Lupus. 2009;18:1198–1204.
- West TW. Transverse myelitis-a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167-177.
- Sellner J, Luthi N, Schupbach WMM, Gebhardt A, Findling O, Schroth G, et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord. 2009;47:312–317.
- Stephen L. Hauser, Allan H. Ropper. From Diseases of the spinal cord. In: Longo, Fauci, Kasper, Hauser, Jameson, Loscalzo (eds). Harrison’s Principles of Internal Medicine. Volume 2. 18th Edition. New York; 2012: pp.3373-3374.
- Ko HY, Kim W, Kim SY, Shin MJ, Cha YS, Chang JH, et al. Factors associated with early onset post-traumatic syringomyelia. Spinal Cord. 2012;50:695–698.
- Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505.
- Banwell B, Tenembaum S, Lennon VA, Ursell E, Kennedy J, Bar-Or A, et al. Neuromyelitis optica-IgG in childhood inflammatory demyelinating CNS disorders. Neurology. 2008;70:344–352.
- Andronikou S, Albuquerque-Jonathan G, Wilmshurst J, Hewlett R. MRI findings in acute idiopathic transverse myelopathy in children. Pediatr Radiol. 2003;33:624–629.
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489.
- Bakshi R, Kinkel PR, Mechtler LL, Bates VE, Lindsay BD, Esposito SE, et al. Magnetic resonance imaging findings in 22 cases of myelitis: comparison between patients with and without multiple sclerosis. Eur J Neurol. 1998;5:35–48.
- Choi KH, Lee KS, Chung SO, Park JM, Kim YJ, Kim HS, et al. Idiopathic transverse myelitis: MR characteristics. Am J Neuroradiol. 1996;17:1151–1160.
- Sellner J, Hemmer B, Muhlau M. The clinical spectrum and immunobiology of parainfectious neuromyelitis optica (Devic) syndromes. J Autoimmun. 2010;34:371–379.
- JL Kitley, MI Leite, JS George, Palace JA. The differential diagnosis of longitudinally extensive transverse myelitis. MultScler. 2012;18(3):271-285.
- Blank M, Barzilai O, Shoenfeld Y. Molecular mimicry and autoimmunity. Clin Rev Allergy Immunol. 2007;32:111–118.
- Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157.