6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffe43b340000007e06000001000600
6go6ckt5b5idvals|3077
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Invasive fungal infections (IFI) of the genitourinary tract are increasingly being recognized. This is perhaps due to expanding population of high-risk patients, use of novel pharmacotherapies and immunosuppressants [
1], as well as due to increasing number of genitourinary procedures. This may also be attributed to the advances in the radiological and microbiological diagnostic methods. IFI of genitourinary tract have mostly been described with yeast like
Candida, Cryptococcus, Trichosporon and with mucorales [
2,
3]. Aspergillosis is uncommonly been described in the urinary tract and often, seen in immunocompromised individuals. In this report, we present a case of urinary tract aspergillosis in an immunocompetent individual who acquired it following double-J (D-J) stenting and review urinary tract aspergillosis.
Case Report
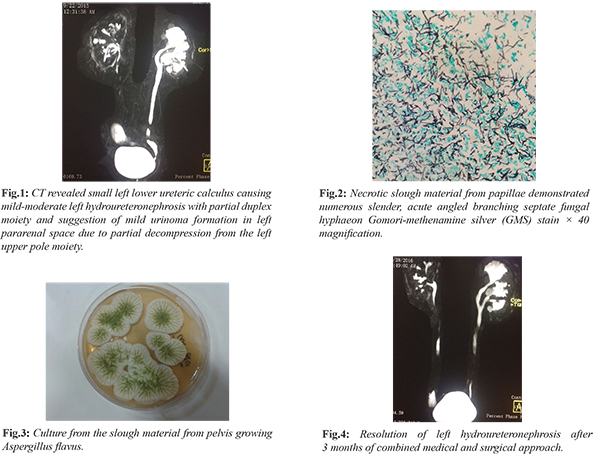
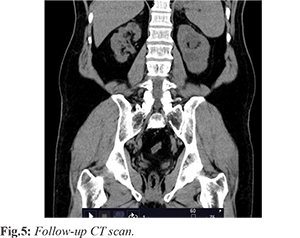
A 59-year-old gentleman with well controlled type-2 diabetes mellitus presented to the emergency department of our hospital with fever, abdominal pain and acute kidney injury (AKI). His laboratory evaluation showed hemoglobin (Hb) 13.6 gm/dL, total leucocyte count (TLC) 8,250 cells/cm3 with 85% neutrophils with platelets 1,39,000 lakh/cu.mm and raised creatinine (4.60 mg/dL). Blood and urine culture grew carbapenem resistant Klebsiella pneumoniae (CR KP) suggestive of complicated urinary tract infection (UTI) with bacteremia. The patient was initiated on polymyxin-E combination therapy. Ultrasonography (USG) abdomen revealed left grade-2 hydroureteronephrosis with right renal concretions. Computed tomography (CT) revealed small left lower ureteric calculus causing mild-moderate left hydroureteronephrosis [Fig.1]. On the polymyxin and meropenem combination therapy, patient clinically improved and repeat blood culture yielded no growth but the patient was persistently febrile. There was a progressive increase in peripheral TLC to 16860 cells/cm3 and increase in C-reactive protein (CRP) to 321 mg/L. Infectious Diseases (ID) consultation was sought at this point. History revealed that patient had earlier undergone a urologic procedure for stone removal with double-J (DJ) ureteral stent placement in the left kidney 7 months prior to admission, in another institute. A few days after the procedure, he developed fever with flank pain and was admitted due to complicated UTI with CR KP. USG KUB revealed an evolving abscess in the left kidney with right kidney pyelonephritis. The 99m Tc-DTPA renal function study was suggestive of impaired perfusion and cortical uptake in the right kidney and hydronephrosis, obstruction and impaired cortical function of the left kidney. In view of the hydronephrosis, a percutaneous drain was put in the left kidney and the bilateral DJ stents were removed and replaced. Cultures were not repeated at this point. A magnetic–resonance (MR) urography done a month later showed filling defects and bilateral renal cortical cysts. Cystoscopy with bilateral retrograde pyelogram with ureteroscopy and bilateral nephroscopic removal of slough material was done. Both the right and left renal calyceal slough material, on histopathology showed amorphous material along with numerous fungal hyphae which were slender, septate with acute angled branching [Fig.2]. Culture grew Aspergillus flavus [Fig.3]. The patient subsequently received voriconazole for 2 months. A repeat imaging was not performed before stopping the antifungal therapy. Subsequently, the patient was asymptomatic for subsequent 3 months until when he got admitted again with complicated UTI with CR KP. In view of the left-sided grade II hydroureteronephrosis, a percutaneous nephrostomy (PCN) tube was placed. The cultures and histopathology from the nephrostomy specimen were negative this time for Aspergillus. However, based on the history, diagnosis of relapse of urinary tract aspergillosis was considered. He was initiated on amphotericin-deoxycholate (AmB-d) (1.0 mg/kg/day) and 5-flucytosine (5-FC) in renally modified doses. The patient responded well to the treatment and became afebrile. The PCN was removed after 2 months followed by the removal of the DJ stents. The combination antifungal therapy was administered for a period of 3 months with close monitoring of the renal function and was discontinued following clinical and microbiological cure along with radiological stability [Fig.4]. The patient has been doing well at 5-year follow up [Fig.5].
Discussion
The incidence of fungal infections of the genitourinary tract is gradually increasing. This may be attributed to the increased use of antibiotics, immunosuppressants, instrumentation, and increasing use of indwelling catheters along with improvement in the diagnostic techniques. Fungal infections of the GU tract have been described most commonly with
Candida spp., Trichosporon spp., Cryptococcus spp., Histoplasmosis spp., Blastomyces spp. and mucormycosis [
2].
Aspergillosis is a life-threatening fungal infection which is usually encountered in severely immunocompromised patients. Common presentations include sinus and lung infections following inhalation of conidia. Historically, urinary tract IA is an uncommon entity. Infection may manifest as renal parenchymal involvement with abscess formation as a part of disseminated disease due to hematogenous seeding of Aspergillus to multiple organs including kidneys. This is described in severe immunocompromised patients [
4,
5]. A few cases of primary renal aspergillosis have been described in patients with human immunodeficiency virus (HIV) infection, intravenous drug users (IVDU) [
4], or renal transplantation. Recently primary GU IA has been described in a receiving venectoclax for treatment of chronic lymphocytic leukemia (CLL) [
1]. Renal pelvis involvement can manifest as either bezoar and casts formation (aspergilloma or a fungus ball) or invasive aspergillosis with subsequent obstructive symptoms. Ascending infection from the lower urinary tract is less frequent [
6]. Only a few cases have been reported following previous ureteral instrumentation [
7] and obstructive uropathy [
4] in immunocompetent patients. Prostrate infection by Aspergillus is reported even more rarely. In our immunocompetent patient, the probability of Aspergillus being introduced into the urinary tract during placement of D-J stent in the ureter is high.Aspergillus is a ubiquitous fungus. Infection may have resulted from fungal spores contaminating the instruments (D-J stent, cystoscope or ureteroscope) due to suboptimal infection control practices in smaller healthcare facilities. The underlying diabetes probably facilitated the localized infection.
The possibility of urinary tract aspergillosis is usually ignored in the differential diagnosis since it is uncommon and has a non-specific clinical presentation. Routine urine cultures may not grow the Aspergillus, which also delays the diagnosis. An intravenous pyelogram is usually suggestive of filling defects, hydronephrosis, and a delayed or absent pyelogram. Retrograde pyelography may be a more reliable investigation for diagnosing the fungus ball. Serum Galactomannan (GM) may be useful in diagnosis of renal aspergillosis if it is a part of disseminated disease in neutropenia or severely immunocompromised patients but not in immunocompetent patients. Diagnosis of post-urosurgical aspergillosis is often established with fungal cultures and histopathological examination of the specimen from renal pelvis as in our case. Cultures may need to be kept for prolonged incubation in the laboratory.
Treatment of aspergillosis with pelvis involvement is challenging. Oral mold-active antifungal agents, such as voriconazole, isavuconazole and posaconazole might achieve effective concentrations in the renal parenchyma but achieve very minimal concentrations in the urine. Liposomal amphotericin B (L-AmB) and echinocandins also has limited urinary concentration in contrast to Amb-d and 5-FC. 5-FC has limited activity against Aspergillus when tested at pH7. However, it has 4000-fold activity at pH5 due to the activity of the gene fcyB that encodes a permease needed for entry of 5-FC into the fungal cell [
8]. Effective source control including nephrectomy may be needed for patients who fail to respond to medical and endourological procedures. Our patient had responded well after the effective nephroscopic removal of the necrotic material from pelvis but perhaps had a relapse owing to ineffective concentration of voriconazole in the urine. He responded well to the synergistic combination therapy with AmB-d and 5-FC.
Conclusion
Post-urosurgical aspergillosis is life threatening rare manifestation of invasive aspergillosis. It requires a high index of clinical suspicion particularly in patients presenting with UTI and obstructive uropathy following an invasive genitourinary procedure. This has implications for sending appropriate intra-operative specimen for fungal cultures as well besides the histopathology. Successful outcome depends upon a combined surgical and medical approach with good effective source control and appropriate mold-active antifungal agents achieving effective urinary levels for right duration. Good infection control practices with high-level disinfection with 2% glutaraldehyde or plasma sterilization needs to be emphasized for prevention of nosocomial fungal infections.
Contributors: NG, AJV: manuscript writing, patient management; PG: manuscript editing, surgical management; DG: manuscript editing and histopathology; KA, RS: critical inputs into the manuscript. AJV will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study.
Funding: None; Competing interests: None stated.
References
- Albin OR, Soper N, Khurana I, Kauffman CA. Invasive genitourinary aspergillosis in a patient with chronic lymphocytic leukemia treated with venetoclax: Case report and review of the literature. Open Forum Infectious Diseases. 2019;6(11).
- Sobel JD, Vazquez JA. Fungal infections of the urinary tract. World J Urol. 1999;17(6):410-414.
- Kauffman CA. Diagnosis and management of fungal urinary tract infections. Infect Dis Clin North Am. 2014;28(1):61-74.
- Hope WW, Walsh TJ and Denning DW. The invasive and saprophytic syndromes due to Aspergillus spp. Med Mycol. 2005;43 Suppl 1:S207-238.
- Segarra J, Chéchile G, Balcells FS. Renal abscess due to Aspergillus fumigatus in a patient with the acquired immunodeficiency syndrome. Urol Int. 1995;55(1):60-62.
- Nabi G, Cook J, N’Dow J, McClinton S. Outcomes of stenting after uncomplicated ureteroscopy: Systematic review and meta analysis. BMJ. 2007;334(7593):572.
- Rao P. Aspergillus infection in urinary tract post ureteric stenting. Indian J Med Microbiol. 2015;33(2):316-318.
- Gsaller F, Furukawa T, Carr PD, Rash B, Jöchl C, Bertuzzi M, et al. Mechanistic basis of pH-dependent 5-flucytosine resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 2018;62(6):e02593-17.