6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffb010360000007608000001001100
6go6ckt5b5idvals|3102
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Gastric neuroendocrine tumors (G-NETs) originate from the enterochromaffin like (ECL) cells of the gastric mucosa form less than 1% of all gastric tumors and about 6-8% of all neuroendocrine tumors (NETs) [
1]. G-NETs have been classified into three subtypes. Type I arise in the presence of chronic atrophic gastritis and is the most common (70-80%). Type II is seen in association with gastrinomas and multiple endocrine neoplasia (MEN) syndrome (5-10%). Type III is sporadic in occurrence (10-15%) and has the highest malignant potential [
2-
4]. G-NETs are mostly asymptomatic or can present with gastrointestinal (GI) bleeding, anemia, fatigue, epigastric pain and vomiting [
3,
5]. They are seen as small to large, single to multiple polypoidal lesions on endoscopy.
This is an unusual case of G-NET presenting as an ulcer along the greater curvature of the stomach presenting with pain and misdiagnosed as chronic gastritis on endoscopic biopsy.
Case Report
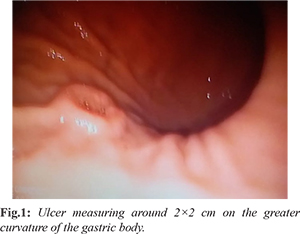
A 52-year-old female presented to our hospital with complaints of epigastric pain for 1 year. She did not have any known co-morbidities. Her general and systemic physical examination was unremarkable. She underwent an esophagogastroduodenoscopy (EGD) which revealed chronic gastritis and an ulcer measuring around 2×2 cm on the greater curvature of the gastric body [Fig.1]. An adequate biopsy sample was obtained from the ulcer and histopathological examination of the same showed features of chronic gastritis. We did not have facilities for endoscopic ultrasound (EUS) and EUS guided biopsy at our centre. A contrast enhanced computerized tomographic (CECT) scan of the abdomen did not reveal any significant abnormalities. Based on the aggressive appearance of the ulcer and clinical history of patient, intractable ulcer to medical therapy for a long duration and with a high degree of suspicion for malignancy, decision was taken to perform gastric resection. She underwent distal gastrectomy with Billroth II anastomosis.
Gross examination showed an ulcer of 1.3 cm in diameter along the greater curvature of stomach [Fig.2]. Histopathological examination showed strips of gastric mucosa with underlying tissue showing tumor cells, with scanty cytoplasm and round to oval nuclei with stippled chromatin, arranged in nests and organised pattern [Fig.3]. The mitotic index was 0-2/10 HPF. Immunohistochemical analysis revealed cells expressing synaptophysin and CD56 positivity and Ki-67 index up to 5%. These features were consistent with neuroendocrine tumor (WHO grade 2).
The post-operative recovery was uneventful and patient was referred to medical oncology for adjuvant chemotherapy. She was recurrence free and tolerating the treatment well at 6 months follow up.
Discussion
Neuroendocrine tumors are epidermal tumors characterized by both endocrine and neural differentiation and these can arise from any part of the body. Gastric NETs (G-NETs) are known to originate from the enterochromaffin like (ECL) cells of the gastric mucosa [
1]. G-NETs tend to be asymptomatic in many cases and are detected incidentally on endoscopic evaluation. They can present with GI bleeding, anemia, fatigue, epigastric pain and vomiting. Rarely they can present with carcinoid syndrome due to histamine release [
3,
5]. They are seen as small to large, single to multiple polypoidal lesions on endoscopy. Work-up for diagnosis and staging of G-NETs include EGD, endoscopic ultrasound and CECT scan to accurately determine the extent and depth of the lesion and lymph nodal involvement. Newer modality of imaging includes somatostatin receptor scintigrapy (SRS) with labelled octreotide that can help detecting primary as well as metastasis [
3,
4]. Chromogranin A is the most useful plasma marker of NET used for follow up [
3].
On histopathology, G-NETs appear as small round to polygonal cells with central nuclei and fine chromatin, small nucleoli and varying amount of cytoplasm with few mitosis [
3]. Synaptophysin and chromogranin A staining is used for neuroendocrine phenotype confirmation. Ki-67 is recommended as a proliferative index marker for prognostication and management [
5,
6]. Factors such as perineural and angioinvasion, Ki-67 index of >3%, high mitotic count and cellular atypia are features of a malignant variety of NET [
3,
7]. WHO classifies G-NETs into low (G1), intermediate (G2) and high grade (G3) based on mitotic count and Ki-67 index [
7,
8].
The European Neuroendocrine Tumor Society (ENETS) guidelines recommend endoscopic resection of superficial type I G-NETs <2 cm [
6,
9]. Type II tumors tend to be more aggressive and management consists of endoscopic resection for early tumors smaller than 2 cm and polypectomy or gastric resection for larger and multiple tumors. Type III are treated with total or partial gastrectomy [
3,
4]. Gastric resection is the gold standard of treatment for resectable G-NETs and has been shown to have survival benefit even among patients with metastatic disease [
1]. Metastatic tumors presenting with poorly differentiated histology are treated with platinum based chemotherapy [
4]. Recent literature also describes type IV G-NETs which are large in size, poorly differentiated on histology and have a very high malignant potential with poorest prognosis of all. These are treated with radical surgery and chemotherapy [
5,
10].
In our case, typical endoscopic findings of NETs such as polypoidal growth with or without ulceration were not observed. Even endoscopic biopsy did not yield the correct diagnosis. We were limited by absence of EUS guided FNAC facilities at our centre and the patient underwent surgery based on clinical judgement. This case highlights an atypical presentation of G-NET with an ulcerated lesion which is rarely seen.
Conclusion
Gastric neuroendocrine tumors are rare and mostly present with polypoidal lesions. Here we report a case of an ulcerated G-NET which was managed with gastric resection with favourable outcome. This presentation should be considered with high index of suspicion when evaluating a gastric ulcer clinically suggestive of neoplastic etiology.
Acknowledgment: The authors would like to acknowledge the contribution of Pathologists at the Nandikur laboratory, Mangalore, Dr Rao and Dr Upadhaya for providing the histopathology images.
Contributors: KP. BV, RC and SRB were all involved in patient care and contributed to conception of the article. RC and BV did the literature review and wrote the first draft of the manuscript. SRB and KP proof read the manuscript and provided significant insights. RC will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study.
Funding: None; Competing interests: None stated.
References
- Yang Z, Wang W, Lu J, Pan G, Pan Z, Chen Q, et al. Gastric neuroendocrine tumors (G-Nets): Incidence, prognosis and recent trend toward improved survival. Cell Physiol Biochem. 2018;45(1):389-396.
- Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: A clinicopathologic study. Gastroenterology. 1993;104(4):994-1006.
- Christopoulos C, Papavassiliou E. Gastric neuroendocrine tumors: Biology and management. Ann Gastroenterol. 2005;18(2):127-140.
- Kwon D, Nakakura E, Bergsland E, Dai SC. Gastric neuroendocrine tumors: management and challenges. Gastrointest Cancer Targets Ther. 2017;7:31-37.
- Dias AR, Azevedo BC, Alban LBV, Yagi OK, Ramos MFKP, Jacob CE, et al. Gastric neuroendocrine tumor: review and update. Arq Bras Cir Dig. 2017;30(2):150-154.
- Perren A, Couvelard A, Scoazec J-Y, Costa F, Borbath I, Delle Fave G, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Pathology - diagnosis and prognostic stratification. Neuroendocrinology. 2017;105(3):196-200.
- Klöppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162-166.
- Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707-712.
- Gluckman CR, Metz DC. Gastric neuroendocrine tumors (Carcinoids). Current Gastroenterol Rep. 2019;21:1-7.
- Li TT, Qiu F, Qian ZR, Wan J, Qi XK, Wu BY. Classification, clinicopathologic features and treatment of gastric neuroendocrine tumors. World Journal of Gastroenterology. 2014;20:118-125.