6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff2449370000006007000001000a00
6go6ckt5b5idvals|3114
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Epilepsia partialis continua (EPC) refers to a predominantly motor presentation of partial seizure dominated by continuous/repetitive jerky (mostly clonic/myoclonic) movements of a focal region of the body (limbs, facial, lingual or abdominal muscles) which are prolonged lasting from minutes to days. A sensory variant of this entity has also been described. Etiopathogenesis of this ictal phenotype is not completely understood while the culprit anatomical substrate could be cortical or subcortical [
1]. There have been sparse reports of EPC in stroke but never has it been cited as presenting manifestation of acute stroke. This unusual presentation and its mechanisms are elaborated in this report.
Case Report
A 88-year-old hypertensive and diabetic gentleman was noticed to have repetitive jerky movements of the right upper limb for the past two days. The jerking was nearly continuous, involuntary and seemed to flow from distal to proximal limb but did not produce much shoulder movements. The movements persisted during sleep and hampered his daily routine to a significant degree. Since past 24 hours, he also felt difficulty in gripping with right upper limb, but it was difficult to discern as the jerks had almost rendered the hand useless. He had no past history of tremor, seizures, transient ischemic attack (TIA), stroke or coronary artery disease.
On examination, he had normal vitals; systemic examination was unremarkable; no carotid bruit. His left upper limb displayed near-continuous jerks reminiscent of clonic movements which seemed to vary in intensity and distribution (in terms of muscle groups involved); they had a distal to proximal-flowing rather than marching character sometimes giving the impression of one movement riding on another. There was associated grip weakness (30%) and right upper limb weakness (difficult to ascertain because of jerks). Right lower limb power was 4/5 without any sensory deficit or cerebellar signs.
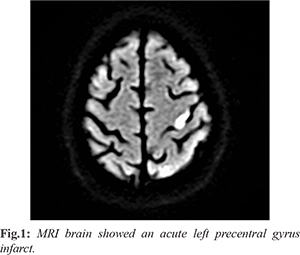
A clinical diagnosis of epilepsia partialis continua was made which was supported by the presence of low voltage fast activity in the left frontocentral channels on electroencephalogram (EEG). Considering the weakness, a structural pathology was suspected, and MRI brain showed an acute left precentral gyrus infarct [Fig.1]. Magnetic resonance angiography (MRA) also showed atherosclerotic narrowing of the left supraclinoid internal carotid artery. The seizures were extremely difficult to control and multiple antiepileptic drugs (AED) were used over the next 48 hours (phenytoin, lacosamide, brivaracetam, eslicarbazepine, clobazam and zonisamide) with only 50-60% decline in the seizures. However, in the next 24-48 hours, he developed significant weakness of the upper limb (1/5 power) as well as the lower limb (2/5 power) on the right side. The deterioration of the power seemed to be inversely related to the seizure frequency and by the time the stroke was completed the seizures had come to a halt. Drugs were rapidly tapered over the next two days in view of the patient turning drowsy. He was stabilized on a combination of eslicarbazepine (400 mg), lacosamide (200 mg) and clobazam (5 mg) at the time of discharge. When seen on the last follow up (6 months after the event), he was on two AEDs with no further seizures.
Discussion
Epilepsia partialis continua, often referred to as a variant of cortical myoclonus an entity involving arrhythmic focal jerky movement of a part of the body can have both cortical and subcortical roots of origin. Usually resistant to multiple anti-epileptic drugs (AED)s, they are often tagged as refractory epilepsy. They may continue on for weeks thereby adding on to the psychosocial debilitation for the patient and the caretakers. Classically seen in relation to structural lesions, vascular malformations and infective masses, EPC is also seen in conjunction with chronic strokes. However, EPC as a presenting feature of acute ischemic stroke is seldom reported [
2]. While some studies had hypothesized it to a cortical overstimulation, there are others which highlighted the reflex component in it. Absence of typical epileptiform pattern in EEG, with enhanced radio-isotope uptake in nuclear imaging had also pointed to a subcortical origin most likely the thalamus. The intense inter and intra-hemispheric spread enables the transition to other seizure patterns. The present report describes a near continuous jerking of limbs with a clearcut ictal correlate which excludes the possibility of a hypoperfusion related limb-shaking transient ischemic attack.
Cockrell et al. in a case series reported 16 cases of EPC of varied origin. The commonest cause identified was Rasmussen encephalitis in children and atherosclerotic vascular disease in adults. Various theories like mitochondrial dysfunction, neuronal migration disorder, etc. had been proposed for the same. One of the patients had jerky movements in the hemiparetic limb, with no voluntary movement or response to cranial magnetic stimulation, suggesting the existence of a pathway independent of the physiological cortico-spinal or motor pathway. Two similar reports of EPC in hemiparetic limb were also reported by Thomas et al. Bentes et al. in their report described stroke as an important cause for EPC. In the 151 patients he studied, 2 (<2%) developed EPC post-stroke, one as an acute complication and the other chronic. Involuntary movement succeeding stroke are also described, but usually associated with basal ganglia, midbrain, pontine or medullary lesions [
3]. In a nation-wide register-based investigation, Zelano et al. concluded that nearly 1.5% of the strokes were preceded by seizures within the last 10 years. Later defined as herald seizures, majority were associated with hemorrhagic lesions than infarcts in younger population reason for which remain largely unknown [
4].
The ischemic penumbra region with its property of peri-infarct depolarisation, is often considered the substrate for early stroke seizures [
5]. This region could serve as a potential epileptogenic substrate because of the following reasons: (i) increased membrane permeability leading to release of excitatory neuro-transmitters like glutamate [
6], (ii) ion- pump dysregulation due to lack of ATP resulting in increased availability of intracellular sodium which in turn facilitates depolarization [
7], (iii) dysregulation of calcium homeostasis can stimulate activation of pathways which generate free radicals and cause mitochondrial dysfunction. However, the impact of these alterations on seizure initiation and propagation are unclear [
8], (iv) cortical hyperexcitability and tendency for intra- and inter-hemispheric spread [
9].
Conclusion
The critical metabolic pathways which are operative in the initial phases of the stroke-associated epilepsy is discussed with the report.
Contributors: SS, NK: manuscript writing, patient management; MJ, AKP: manuscript editing, patient management; SP, SC: critical inputs into the manuscript. SS will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study.
Funding: None; Competing interests: None stated.
References
- Mameniškiene R, Wolf P. Epilepsia partialis continua: a review. Seizure. 2017;44:74-80.
- Guerrini R. Physiology of epilepsia partialis continua and subcortical mechanisms of status epilepticus. Epilepsia. 2009;50:7-9.
- Bentes C, Franco AC, Peralta AR, Viana P, Martins H, Morgado C, et al. Epilepsia partialis continua after an anterior circulation ischaemic stroke. European J Neurol. 2017;24(7):929-934.
- Zelano J, Larsson D, Kumlien E, Åsberg S. Pre-stroke seizures: a nationwide register-based investigation. Seizure. 2017;49:25-29.
- Camilo O, Goldstein LB. Seizures and epilepsy after ischaemic stroke. Stroke. 2004;35:1769-1775.
- Alberti A, Paciaroni M, Caso V, Venti M, Palmerini F, Agnelli G. Early seizures in patients with acute stroke: frequency, predictive factors, and effect on clinical outcome. Vascular Health and Risk Management. 2008;4(3):715.
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nature Medicine. 2008;14(5):497-500.
- Zhao Y, Li X, Zhang K, Tong T, Cui R. The progress of epilepsy after stroke. Current Neuropharmacology. 2018;16(1):71-78.
- Taxin ZH, Neymotin SA, Mohan A, Lipton P, Lytton WW. Modeling molecular pathways of neuronal ischemia. Prog Mol Biol Transl Sci. 2014;123:249-275.
- Cockerell OC, Rothwell J, Thompson PD, Marsden CD, Shorvon SD. Clinical and physiological features of epilepsia partialis continua: cases ascertained in the UK. Brain. 1996;119(2):393-407.