6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffe4a6370000004b0a000001000100
6go6ckt5b5idvals|3118
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
This report describes a case where the diagnosis of an ileal neuroendocrine tumor (NET), with local mesenteric node involvement, was initially misdirected and worked up as a hematological presentation. Small bowel neuroendocrine tumors (SBNETs) have a reported incidence between 0.32-1.12/100,000 in the literature, with most originating from the terminal ileum [
1,
2]. They are derived from enterochromaffin cells and are characterised by their indolent growth [
1]. Patients with SBNETs most commonly complain of non-specific abdominal pain with diarrhoea and skin flushing being the cardinal symptoms for functional tumors [
1]. Lymph node involvement is common with SBNETs, with reported rates between 46-98% [
3]. In particular, mesenteric nodal metastases from SBNETs have often been described to be considerably larger than the primary tumor [
3].
The 2016 edition of the European Neuroendocrine Tumour Society Consensus Guidelines recommend initial computed tomography (CT) and/or magnetic resonance imaging (MRI) followed by Gallium DOTATATE positron emission tomography (PET) with concurrent CT imaging for the search of SBNETs [
1]. Nodal involvement and distant metastasis is more regularly detected through the initial CT/MRI, with the primary tumor being identified less frequently [
1]. Colonoscopy is also recommended in the search for a primary SBNET in the distal ileum and to rule out synchronous colorectal cancer [
1].
We present a case where the significant size of the involved mesenteric node masked the underlying diagnosis of an ileal NET. Subsequently, the patient was investigated for underlying hematological and infective causes of her mesenteric lymphadenopathy, delaying key somatostatin receptor imaging. This case demonstrates the importance of recognising SBNET with local nodal involvement as a differential diagnosis in a patient with mesenteric lymphadenopathy.
Case Report
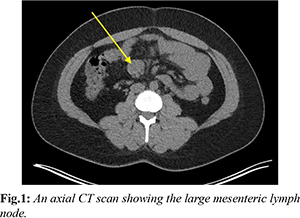
A 36-year-old female presented to the emergency department with a 3-month history of intermittent epigastric pain that was gradually worsening and not responding to simple analgesia. This was associated with nausea, vomiting and hot flushes. Prior to this, she was being investigated by the hematology team for lymphadenopathy and low ferritin levels. CT scan of her abdomen and pelvis at that time demonstrated a small bowel mesenteric mass measuring 44×16×35 mm in size that had radiological density consistent with lymph nodes [Fig.1]. An initial diagnosis of lymphoma was given after multiple blood investigations for infection were negative. A positron emission tomography fluorodeoxyglucose (FDG-PET) scan showed low avidity in both the enlarged mesenteric nodes and subsequent bone marrow aspirate trephine (BMAT) was negative for hematological malignancies or disorders.
A laparoscopic lymph node biopsy of the mesenteric lymph nodes revealed metastatic neuroendocrine neoplasm with suspicions of the gastrointestinal tract or ovarian origin. NET specific investigations such as tumor markers and functional imaging with PET gallium DOTATATE (Ga-DOT PET) showed focal intense avidity over a loop of small bowel in the mid-lower abdomen, the site of the previous known mesenteric mass [Fig.2]. Chromogranin A level was also raised at 133 ng/mL. She was then commenced on a somatostatin analogue (octreotide) which significantly improved her symptoms.
She underwent a laparotomy to resect the affected small bowel and mesenteric lymph nodes. Histopathology showed grade 1 neuroendocrine tumor of ileum with a Ki 67 index of <1%. Synaptophysin was positive and the lymph node showed neoplastic activity. Her post-operative progress was complicated by prolonged ileus. She subsequently recovered and was discharged home.
Discussion
NETs are frequently found in the gastrointestinal tract (62-67%) [
4]. 45-65% of gastrointestinal NETs arise from the midgut, particularly the terminal ileum, with 10-20% of patients are found to have metastatic disease on initial presentation [
4,
5]. NETs can be classified as functioning or non-functioning based on the presence or absence of clinical symptoms that arise from the hypersecretion of metabolically active substances [
6]. This constellation of symptoms is termed carcinoid syndrome and they include skin flushing, diarrhoea and sweating [
7,
8]. These symptoms are usually present in 6-30% or when liver metastasis is present [
7,
8]. However, according to Scherubl (2010), patients with ileal NETs usually present symptomatic from the tumor or carcinoid syndrome [
7]. Lymph node metastases are relatively common in ileal NETs with peritoneal seeding and distal metastases occurring in 20-60% of patients [
7]. Our patient initially had typical symptoms of an ileal NET – abdominal pain and episodes of flushing. However, an external CT scan of her abdomen and pelvis demonstrated a 44×16×35 mm mesenteric soft tissue mass which was thought to be a lymph node and no other suspicious lesions. This led to her being investigated for causes of lymphadenopathy given that NETs are rare and there was no primary tumor seen on the CT scan. Mesenteric masses are usually lymphatic in origin, reflecting that they are usually haematological [
5]. Compared to NETs of the gastrointestinal system, primary mesenteric NETs are very rare and are usually metastatic tumors [
9]. Lymphomas can manifest as nodal and/or extra-nodal, non-Hodgkin’s lymphoma commonly (45%) involves the abdominal mesentery [
10]. Nodal disease may present as a large (either singularly enlarged or multiple lymph nodes fused) uniform density lesion on CT scan similar to the radiological finding of our patient [
10]. With extra-nodal disease, though rare (1-2% of primary gastrointestinal tumors), lymphoma can present as a primary tumor of the small intestine where patients may experience non-specific abdominal symptoms such as colicky abdominal pain, nausea and vomiting [
11].
Diagnosing NETs require results from blood investigations and both diagnostic and functional imaging [
12]. Chromogranin A is a biomarker that has high sensitivity (70-100%) for detecting both functional and non-functional NETs [
8,
12]. Chromogranin A serum values are raised in 60-100% of patients with NETs [
8]. Diagnostic imaging such as CT or MRI is usually the first modality and provides good anatomical delineation of the tumor and evidence of metastases [
13]. This is not true for our patient as the primary lesion was not visualized on initial scans. The diagnosis was only confirmed on histopathology after a laparoscopic procedure to surgically excise the mesenteric lesion. CT scans with intravenous (IV) contrast usually have a mean sensitivity of 73% for detecting primary NET [
14]. However, small sub-centimetre lesions can still be missed [
14]. This may explain why the ileal lesion was not identified on the patient’s initial CT scan. The FDG-PET scan done revealed low avidity in two mesenteric lymph nodes. It also showed activity in the left ovary and in a solitary right thyroid nodule which further confounded the diagnosis. FDG-PET uses radio-labelled glucose which is significantly up-regulated in malignancies such as lymphomas [
15]. DOTATATE PET scan utilizes Gallium-68 DOTA peptide (68Ga-DOTATATE), a selective somatostatin receptor tracer (SSTR) which has a significant affinity to the SSTR receptors [
14,
16]. 68Ga-DOTATATE PET/CT scan is the preferred choice of scan when a diagnosis of NET is clinically suspected as it has a sensitivity of 93% and specificity of 91% for detection of the primary tumor [
14]. It also provides accurate staging to allow clinicians to determine the next appropriate clinical management. It is reported that up to 10-22% of NETs have unknown primary tumor site [
14]. Prasad et al. concluded that an unknown primary tumor was detected by 68Ga-DOTATATE in 59% of the sample size after an ultrasound, CT, MRI and endoscopic ultrasound were performed [
17]. The prospective study by Shell et al. demonstrated the advantage of Ga-DOT PET identifying NETs in patients who had no biochemical or radiological evidence of the disease [
16]. Interestingly, there is a role for FDG-PET in NET. Emerging evidence has shown that FDG-PET can be used for high-grade aggressive tumors and has a correlation between high FDG uptake and poorer outcomes in patients with NET [
15].
Conclusion
This case demonstrates the importance of recognising SBNET with local nodal involvement as a differential diagnosis in a patient with mesenteric lymphadenopathy.
Contributors: DK: manuscript writing, patient management; MW: manuscript editing, patient management; AA, ZD: critical inputs into the manuscript. DK will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study.
Funding: None; Competing interests: None stated.
References
- Niederle B, Pape UF, Costa F, Gross D, Kelestimur F, Knigge U, et al. ENETS consensus guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology. 2016;103(2):125-138.
- Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumours. CA Cancer J Clin. 2018;68(6):417-487.
- Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, et al. The surgical management of small bowel neuroendocrine tumours: consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46(6):715-731.
- Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumours and carcinomas. Neoplasia. 2017;19(12):991-1002.
- Essaoudi MA, Aboulfeth EM, Albouzidi A, Aitali A, Oukabli M. Primary mesenteric neuroendocrine tumour: a case report and review of the literature. SM J Clin Pathol. 2018;3(2):1018.
- Tsoli M, Chatzellis E, Koumarianou A, Koloodi D, Kaltsas G. Current best practice in the management of neuroendocrine tumours. Ther Adv Endocrinol Metab. 2018;10:2042018818804698.
- Scherübl H, Jensen RT, Cadiot G, Stölzel, Klöppel G. Neuroendocrine tumours of the small bowels are on the rise: early aspects and management. World J Gastrointest Endosc. 2010;2(10):325-334.
- Xavier S, Rosa B, Cotter J. Small bowel neuroendocrine tumours: from pathophysiology to clinical approach. World J Gastrointest Pathophysiol. 2016;7(1):117-124.
- Park IS, Kye BH, Kim HS, Kim HJ, Cho HM, Yoo C, et al. Primary mesenteric carcinoid tumour. J Korean Surg Soc. 2013;84:114-117.
- Manzella A, Borba-Filho P, D’Ippolito G, Farias M. Abdominal manifestations of lymphoma: spectrum of imaging features. ISRN Radiology. 2013;483069.
- Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17(6):967-707.
- Raphael MJ, Chan DL, Law C, Singh S. Principles of diagnosis and management of neuroendocrine tumours. CMAJ. 2017;189:E398-404.
- Maxwell JE, Howe JR. Imaging in neuroendocrine tumours: an update for the clinician. Int J Endo Oncol. 2015;2(2):159-168.
- Sanli Y, Garg I, Kandathil A, Kendi T, Baladron-Zanetti MJ, Kuyumcu S, et al. Neuroendocrine tumour diagnosis and management: 68Ga-DOTATATE PET/CT. Am J Roentgenol. 2018;211:266-277.
- Valls L, Badve C, Avril S, Herrmann K, Faulhaber P, O’Donnel J, et al. FDG-PET imaging in hematological malignancies. Blood Rev. 2016;30(4):317-311.
- Shell J, Keutgen X, Millo C, Nilubol N, Patel D, Sadowski S, et al. 68-Gallium DOTATATE scanning in symptomatic patients with negative anatomic imaging but suspected neuroendocrine tumor. Int J Endo Oncol. 2018;5(1):IJE04.
- Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68) Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging. 2010;37:67-77.