6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff646238000000580a000001000100
6go6ckt5b5idvals|3127
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Ramsay Hunt syndrome (RHS) is an infective complication caused by the DNA-based virus varicella zoster virus (VZV), and is typically caused by a reactivation of the dormant virus in either the geniculate ganglion or the 7th cranial nerve. It is characterised by both sensory and motor manifestations given its multimodal function, ranging from a blistering rash over the auditory canal or mouth to a lower motor neuron facial nerve palsy. Other previously described symptoms include tinnitus, hearing loss, nausea, vertigo and nystagmus. RHS is diagnosed by demonstration of active infection through VZV PCR of active lesions or serology to suggest acute infection accompanied by cranial nerve palsies. Cranial nerve involvement beyond the facial nerve, particularly with multiple cranial nerves has been rarely described. We present an unusual presentation of RHS associated with cranial polyneuropathy characterised by a prolonged period of dysphagia.
Case Report
PB, an 83-year-old male presented with a three-week history of right sided facial swelling associated with a rash and occasional coughing with oral intake. He also described a sore throat, hoarse vocal quality and intermittent shortness of breath and 5-day history of vertigo with difficulty walking prior to presentation. On examination, he was noted to have blistering lesions over the right side of his face which extended into his external auditory canal. He had left beating nystagmus on horizontal gaze and was noted to have ataxia. He had no other abnormalities on further cerebellar examination. He was subsequently admitted under general medicine for further assessment. External auditory canal PCR swabs confirmed the presence of varicella zoster virus (VZV). The patient was subsequently confirmed to have RHS. He was not initially commenced on regular antivirals given his delayed presentation.
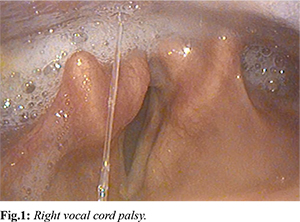
During his admission, a vestibular physiotherapy assessment confirmed right vestibular nerve dysfunction. Examination by the Ear, Nose and Throat specialist team revealed asymmetrical palatal elevation suggesting involvement of the right pharyngeal plexus. He was additionally noted to have an insensate right supraglottis and immobile right vocal cord with poor clearance of secretions suggesting a right recurrent laryngeal nerve palsy [Fig.1]. He was assessed by speech pathologist who placed him nil by mouth (NBM) following an initial flexible endoscopic evaluation of swallowing (FEES) study which revealed a profound sensory and motor dysphagia characterised by reduced pharyngeal contraction and squeeze, reduced epiglottic deflection and reduced airway closure. This resulted in significant post swallow residue throughout the pharynx and poor sensation to pharyngeal residue. Penetration was noted throughout the study and patient presented a high risk of aspiration on his own secretions. A nasogastric tube was required for supplemental feeding and he was not a candidate for dysphagia rehabilitation pending confirmation of aetiology. He was temporarily commenced on intravenous acyclovir 10 mg/kg thrice daily for a period of 3 days whilst awaiting exclusion of intracranial involvement. MRI brain with contrast excluded any intracranial involvement but suggested increased enhancement of the right facial nerve particularly distal to the cisternal portion. A lumbar puncture revealed a mildly elevated CSF protein 640 mg/L with 39×106 cells/L, of which 100% mononuclear, however his CSF VZV PCR returned negative.
A further 2 × FEES studies (5 days and 4 weeks after initial FEES) and a videofluoroscopic swallow study (VFSS) (also 4 weeks after initial FEES) with speech pathology revealed nil to minimal change in his swallow function. Further findings from VFSS highlighted reduced base of tongue propulsion and reduced hyoid movement with subsequently reduced epiglottic deflection and ongoing significant residue with aspiration risk secondary to same. There was only slight improvement noted in his dysphonia (moderate dysphonia). He remained NBM with alternative nutrition and had a percutaneous endoscopic gastrostomy (PEG) tube inserted 5 weeks following his admission. Dysphagia rehabilitation exercises were prescribed daily, just prior to PEG insertion targeting base of tongue movement (Masako manoeuvre) and hyoid movement (shaker/head lift exercise). In addition, following PEG insertion, therapeutic swallow trials following oral cares with half a cup of thin fluids (cold water only) of small, single sips with a right head turn and extra clearing swallow were introduced and allowed throughout the day. This was based off findings in the VFSS to optimise airway closure and pharyngeal clearance. He was discharged home 6 weeks following admission and educated to continue daily dysphagia rehabilitation exercises at home with planned follow up through outpatients.
Upon 3-month outpatient review, he was symptomatic with ongoing vertigo from his persistent right vestibular dysfunction, ongoing right recurrent laryngeal nerve palsy with only a few flickers of movement on the right vocal cord and a mild-moderate dysphonia. A repeat VFSS at this same timepoint revealed mild oral phase deficits and moderate-severe pharyngeal phase deficits a substantial improvement compared to his previous VFSS and subsequently, suitable for commencement of texture modified diet (soft and bite sized) and thin fluids with compensatory strategies (including right head turn). He continued daily dysphagia rehabilitation exercises until his oral intake returned to adequate levels and reduction of strategies required (nil need for head turn to alleviate aspiration signs). Cessation of PEG feeding (excluding water for some hydration) occurred 5 months post onset with the PEG tube then removed at 9 months post onset.
Discussion
RHS resulting in cranial polyneuropathy is extremely rare. Our patient demonstrated RHS with the symptomatic involvement of multiple cranial nerves including VII, VIII, IX, and X. This was evident via the presence of vesicular lesions over the external auditory canal with a facial paresis, accompanied by vertigo from vestibular dysfunction and dysphagia due to motor and sensory cranial nerve deficits. The mechanism for cranial polyneuropathy involvement is unclear, however several hypotheses have been proposed. Firstly, the path of the facial cranial nerve via the general somatic afferent or geniculate nucleus often intermingles with the distribution of other nerves such as the glossopharyngeal, vagus and trigeminal nerves [
1,
2]. Thereby a viral infection of the facial nerve could possibly involve the above-mentioned nerves. Alternatively, glossopharyngeal nerve, vagus nerve, accessory nerve and hypoglossal nerve are supplied by the ascending pharyngeal artery, therefore, a post-infective vasculitis is a potential explanation for the multiple cranial nerve involvement. Based on symptomatology, our case initially presented with facial nerve involvement shortly accompanied by the vestibulocochlear nerve and subsequently by the glossopharyngeal and vagus nerve. This is more suggestive of a direct infection of multiple nerves based on anatomical proximity.
Our patient had severe dysphagia unsafe for oral intake as a complication of his cranial nerve VII, IX and X involvement. This persisted for 3 months following the acute infection and gradually improved with dysphagia rehabilitation. Across literature, dysphagia was evident in only 11 cases of RHS [
3]. Majority of these cases required supplemental feeding through alternative routes such as nasogastric feeding or PEG feeding. Almost all patients with dysphagia eventually recovered enough to allow oral intake except for two cases [
3]. Of those that did recover, the majority sufficiently improved within 2 months of presentation to allow oral intake. Those that took longer to recover were older than 80 years of age. This was consistent in this case, where our patient was older and only recovered sufficiently enough to enable oral intake after a delayed recovery phase lasting 5 months. Poor prognostic factors for recovery of dysphagia include an age above 80 years and the presence of more than three cranial nerves involved [
3]. Dysphagia rehabilitation techniques included the Masako manouvre to increase base of tongue propulsion to posterior pharyngeal wall movement to subsequently reduce pharyngeal residue and the shaker exercise (modified due to vertigo) to assist hyoid movement, epiglottic deflection and clearance through the upper oesophageal sphincter [
4]. The right head turn while swallowing thin fluids successfully alleviated aspiration signs [
5].
Full recovery of patients with polyneuropathy is uncommon. In one study, only 55% of patients with multiple cranial nerve involvement recovered compared to 83% of those with single cranial nerve infections [6]. Our patient still had ongoing vertigo and vocal cord palsy on review 5 months following their initial presentation. This was consistent with previous evidence suggesting approximately 70% with vestibular dysfunction and 60% of those with vagal nerve involvement had at least partial neurological recovery [
7]. Treatment with either topical or oral acyclovir can be commenced when patients present acutely. However, delay in treatment has resulted in poorer prognosis. There is limited evidence of combination treatment with antiviral and steroids resulting in improvement of polyneuropathy [
8]. Swallowing exercises and rehabilitation is the cornerstone for patients with dysphagia. Despite a lack of evidence, swallowing rehabilitation was instrumental in improving nearly half of all patients with RHS and dysphagia [
4].
Conclusion
Our case highlights the need to be vigilant and screen for other cranial nerve involvement in patients with Ramsay Hunt syndrome. This is particularly important given the potential to early treatment which may improve neurological recovery. Furthermore, identifying polyneuropathy allows for intensive rehabilitation to improve focal cranial nerve deficits causing dysphagia. Unfortunately, RHS involvement beyond the facial nerve is associated with poor recovery, particularly when complicated by either vestibular dysfunction, vocal cord palsy or dysphagia. Our case also highlights the complexities in the management of the morbidity associated with these VZV-related complications.
Contributors: TC drafted the manuscript and reviewed the literature; KJ, MK and AT assisted in the diagnosis, management of the patient and have assisted in revisions of the manuscript. AT will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study.
Funding: None; Competing interests: None stated.
References
- de Castro DM, Marrone LC. Neuroanatomy Geniculate Ganglion. Stat Pearls Publishing 2021. Available at https://www.ncbi.nlm.nih.gov/books/NBK555950/ Accessed on August 16, 2022.
- Shim JH, Park JW, Kwon BS, Ryu KH, Lee HJ, Lim WH, et al. Dysphagia in Ramsay Hunt syndrome: A Case Report. Ann Rehabil Med. 2011;35:738-741.
- Lee KM, Jeong HM, Lee, HS, Kim MS. Cranial polyneuropathy in Ramsay Hunt syndrome manifesting severe pharyngeal dysphagia: a case report and literature review. Brain Neurorehabil. 2017;10:e13.
- Daniels SK, Huckabee ML, Gozdzikowska K. Dysphagia following stroke. In: San Diego: Plural Publishing. 2008:pp. 252-254.
- Ha JF. Unilateral vocal fold palsy & dysphagia: A review. Auris Nasus Larynx. 2020;47:315-334.
- Shim HJ, Jung H, Park DC; Lee JH, Yeo SG. Ramsay Hunt syndrome with multicranial nerve involvement. Acta Otolaryngol. 2011;131:210-215.
- Kim YH, Chang MY, Jung HH, Park YS, Lee SH, Lee JH, et al. Prognosis of Ramsay Hunt syndrome presenting as cranial polyneuropathy. The Laryngoscope. 2010;120:2270-2276.
- Babtain FA, Bhatia HS, Assiri AH. Ramsay Hunt syndrome with multiple cranial neuropathies in a liver transplant patient. Neurosciences. 2012;17:262-264.