6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff243d390000009b06000001000b00
Introduction
Primary liver cancers in children under 18 years old account for approximately 0.5-2% of all solid tumors. Among them, hepatoblastoma (HB) is the most common with a worldwide incidence of 1.5 cases per million. HB typically affects boys under 5 years old, representing around 90% of cases [
1-
3]. The histological heterogeneity and rarity of HB can pose diagnostic challenges, even for experienced pathologists, particularly in cases where embryonal epithelial-type HB shares histopathological features with other small round cell tumors (SRCT), such as pancreatoblastoma. The most common site of HB metastasis is the lung, and there are no known reports of HB with metastases to the pancreas in pediatric patients. In this report, we describe the case of an 8-year-old boy with HB who presented with a pancreatic mass.
Case Report
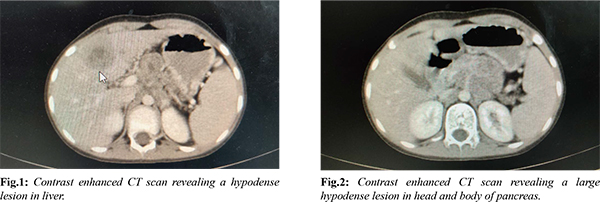
An eight-year-old boy was admitted to the hospital due to abdominal pain. A computed tomography (CT) scan of the abdomen with contrast revealed a bulky pancreas with an ill-defined lesion measuring 2.8×1.8 cm in the body of the pancreas. Additionally, there were multiple space-occupying lesions (SOLs) in the liver, with the largest measuring 4 cm, and multiple enlarged lymph nodes in the para-aortic, celiac, peri-pancreatic, and gastrohepatic regions [Fig.1,2]. The patient's CA19.9 levels were normal. Endoscopic ultrasound (EUS) was used to perform a fine-needle aspiration (FNA) of the pancreatic mass and an image-guided core biopsy of the liver SOL. The EUS-guided pancreatic FNA revealed cellular smears with tumor cells in clusters, nests, sheets, focal acinar patterns, and focal rosettes [Fig.3]. Individual cells were small round cells with inconspicuous nucleoli and scant eosinophilic cytoplasm, without evidence of squamoid nests. The cell block showed similar findings. The possibility of a diagnosis of a small round cell tumor (SRCT), possibly pancreatoblastoma, was considered. The liver biopsy showed benign hepatocytes arranged in trabeculae and tumor tissue in clusters, sheets, and focal acinar patterns. Individual cells had small round nuclei, inconspicuous nucleoli, and scant cytoplasm, without evidence of squamoid nests [Fig.4]. Based on the histopathology findings, differential diagnoses of SRCT were considered, including HB-embryonal epithelial type, metastatic pancreatoblastoma, lymphoma, nephroblastoma, neuroblastoma, and neuroendocrine tumor.
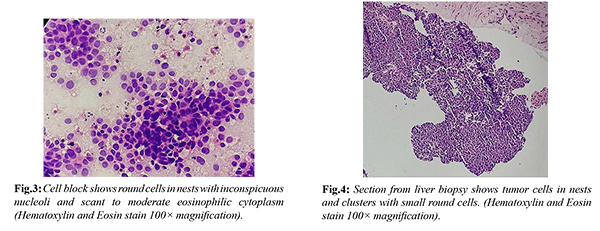
Immunohistochemistry (IHC) was performed on the liver biopsy, with the tumor showing positivity for alpha-fetoprotein (AFP), glypican-3, and L-arginase, suggesting hepatic origin. Synaptophysin and chromogranin were negative, ruling out the possibility of a neuroendocrine tumor or neuroblastoma. Additionally, IHC with beta-catenin showed nuclear positivity, confirming the diagnosis of HB. Other acinar cell markers such as trypsin, chymotrypsin, and lipase were not available. The proliferative index (Ki67) was 80%. IHC on the pancreatic cell block revealed glypican-3 and L-arginase positivity, confirming the diagnosis of embryonal epithelial type HB with metastases to the pancreas. The available treatment options were discussed, and chemotherapy with doxorubicin and cisplatin was advised. Unfortunately, the patient did not return for follow-up.
Discussion
The case report describes a rare pediatric malignancy, hepatoblastoma (HB), which arises from the embryonic liver tissue. In this case, the patient presented with abdominal pain, and imaging studies revealed a bulky pancreas with an ill-defined lesion and multiple space-occupying lesions in the liver, along with enlarged lymph nodes. The diagnosis was established using a combination of imaging, histopathology, and immunohistochemistry.
These tumors can be linked to various syndromes such as Beckwith-Wiedemann syndrome, familial adenomatous polyposis, Li-Fraumeni syndrome, trisomy 18, hemihypertrophy, renal malformation, and other metabolic disorders [
1,
2]. HBs typically have one or more mutations per case [
1-
7]. In around 75% of HBs, mutations impact the Wnt/ß-catenin pathway, resulting in ß-catenin activation [
1]. HBs usually occur as a single mass (unifocal) but can be multifocal. Only one case of multifocal HB has been reported so far [12]. The tumor found in our patient was multifocal. Typically, HB presents as an incidentally detected abdominal mass, weight loss, and anorexia, but less common features include jaundice, vomiting, and diarrhea. Serum AFP is usually elevated and used as a tumor marker [
1,
2]. HBs can be classified by histological subtyping based on their level of cell differentiation. The two morphological subtypes of HB are epithelial and mixed epithelial-mesenchymal. The epithelial type can exhibit an embryonal pattern, fetal pattern, or a mixture of both. The embryonal pattern is the more immature pattern, characterized by small round cells arranged in cords, ribbons, and rosette-like structures. HB with a predominant embryonal pattern can mimic other SRCTs such as Wilms' tumor, Ewing's sarcoma, pancreatic neuroendocrine tumor (PNET), pancreatoblastoma, and neuroblastoma. Diffuse glypican 3 and nuclear beta-catenin positivity aid in the diagnosis of HB [
1,
2,
5]. Metastatic tumors to the pancreas are rare, particularly in the pediatric age group. The most common site of HB metastasis is the lung. HB metastasis to the pancreas is quite uncommon, with only one case reported by Sugina et al. to the head of the pancreas in a 22-year-old man [
7].
Pancreatoblastomas resemble HBs in many aspects. The serum AFP levels may be elevated in both. Both the tumors may harbor mutations involving the Wnt pathway, and be associated with Beckwith-Wiedemann syndrome. Histologically, both show small round cells making the diagnosis challenging on fine needle aspiration cytology and biopsy specimens [
1-
7]. Aruna Prabhu et al. reported a case of pancreatoblastoma presenting with liver metastases, which was misdiagnosed as HB on small biopsy [
8]. This highlights the importance of keeping HB as a differential, in diagnosis and management of liver and pancreatic masses; especially in children.
The primary treatment of choice is surgical resection, and neo-adjuvant chemotherapy with PLADO (cisplatin and doxorubicin) has been used to reduce the size of tumors and increase the resectability of the tumor. However, the outcome for non-resectable cases and recurrent disease is poor. In patients with unresectable non-metastatic disease, liver transplantation may be offered as a novel modality of treatment. These patients must be followed at regular intervals with serum AFP levels, and interval ultrasonography [
3,
5,
9].
Conclusion
Metastasis of HB to the pancreas is an uncommon finding in the pediatric population. The histopathological similarity between embryonal epithelial type HB and small round cell tumors (SRCTs) can create a diagnostic challenge, particularly when imaging reveals involvement of multiple organs, leading to ambiguous results. Therefore, clinicians and pathologists should include HB in their differential diagnosis when liver and pancreas are both affected. This approach will enable accurate diagnosis and prompt treatment initiation.
Contributors: AS: manuscript writing, histopathology; ZN, BF: manuscript editing, histopathology; GVR, DNR: critical inputs into the manuscript and histopathology. AS will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study.
Funding: None; Competing interests: None stated.
References
- Ferrel L, Kakar S. Tumors of liver, biliary tree and gallbladder. In: Fletcher CDM, ed. Diagnostic histopathology of tumors. 4th ed. China: Elsevier Saunders; 2013: p.477-530.
- Ferrel L. Benign and malignant tumours of liver. In: Odze RD, Goldblum JR, ed. Surgical pathology of GI tract, liver, biliary tract and pancreas. 3rd ed. China: Elsevier Saunders; 2015:p.1539-1572.
- Archana B, Thanka J, Sneha LM, Xavier Scott JJ, Arunan M, Agarwal P. Clinicopathological profile of hepatoblastoma: An experience from a tertiary care center in India. Indian J Pathol Microbiol. 2019;62(4):556-560.
- Bell D, Ranganathan S, Tao J, Monga SP. Novel advances in understanding of molecular pathogenesis of hepatoblastoma: A Wnt/ß-Catenin Perspective. Gene Expr. 2017;17(2):141-154.
- Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: An update. Pediatr Dev Pathol. 2020;23(2):79-95.
- Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001;51(9):686-690.
- Sugino K, Dohi K, Matsuyama T, Asahara T, Yamamoto M. A case of hepatoblastoma occurring in an adult. Jpn J Surg. 1989;19(4):489-493.
- Prabhu A, Bhagat M, Ramadwar M, Qureshi SS. Pancreatoblastoma masquerading as hepatoblastoma: A diagnostic dilemma. J Indian Assoc Pediatr Surg. 2016;21(2):84-86.
- Ghaffarian AA, Book L, Meyers RL. Liver transplant for metastatic pancreatoblastoma: 7-year event-free survival after chemotherapy, pancreatectomy, complete hepatectomy, and liver transplant. Pediatric Transplantation. 2018;22(1):13098.