Alok Sharma1, Hemangi Sane2, Amruta Paranjape2,3, Nandini Gokulchandran1, Sushant Gandhi3, Prerna Badhe1
Departments of 1Medical Services, 2Research and Development, 3Neurorehabilitation; NeuroGen Brain and Spine Institute, Navi Mumbai, India.
Corresponding Author:
Miss Amruta Paranjape
Email: amruta.paranjape@live.co.uk
Abstract
Introduction: Ischemic stroke is brain injury due to hypoperfusion leading to sudden neurological symptoms. Despite advances in medicine, prognosis is still grim especially with chronic residual neurodeficits. Autologous bone marrow mononuclear cells (BMMNCs) transplantation is safe and has shown positive outcomes in previous studies of ischemic stroke. Our aim was to study the effect of autologous BMMNCs transplantation in chronic ischemic stroke. Intervention and Results: We treated a case of pontine ischemic infarct three years later, with BMMNCs transplantation followed by standard rehabilitation. Seven months after transplantation he showed significant improvement in spasticity, motor function and voluntary control of upper and lower extremity, static and dynamic sitting and standing balance. Improvement was noted in the scales like Modified Ashworth scale (mAS), Motor Assessment scale (MAS), Disabilities of Shoulder, arm and hand questionnaire (DASH), Modified Rankin Scale (MRS), Berg Balance Scale (BBS) and Brunnstrom Voluntary Control Grading (BVCG). Functional Independence Measure score increased from 59 to 81. BMMNCs exert paracrine effects, neoangiogenesis and trophic effect which facilitate various reparative and regenerative processes in the penumbral area. Conclusion: In this case, the clinical changes exhibited by BMMNCs transplantation even at a chronic stage in stroke substantiate their therapeutic potential. Although a single case report, these findings provide foundation for further research. Larger randomized controlled trials are warranted to study the safety and efficacy.
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff68760b0000008304000001000600 6go6ckt5b5idvals|576 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Ischemic stroke is caused by hypoperfusion of the brain. Despite all medical advances prognosis is still grim and functional outcome is poor. Transplantation of autologous bone marrow mononuclear cells (BMMNCs) improves motor and cognitive deficits in rat mode of stroke [ 1]. Systemic transplantation of BMMNCs in patients with acute stroke is safe and feasible [ 2]. Intrathecal application of BMMNCs has been tested in patients of various neurological diseases for safety and feasibility [ 3, 4]. BMMNCs various neurotrophic factors protect the brain cells from hypoxic and hypoglycemic injuries [ 5]. Natural response to injury is mobilization of BMMNCs, by a stromal derived factor 1 (SDF-1) - CXCR4 chemotactic pathway, from the bone marrow to peripheral blood to the injured brain regions. The mobilization is proportional to extent of injury and number of cells mobilized reduced in chronic stage [ 6].
There is evidence of safety and efficacy of autologous BMMNCs systemic transplantation in acute or sub-acute stroke patients. The effects of autologous BMMNCs intrathecal transplantation in chronic stroke has not been explored. We present the findings in a case of pontine ischemic stroke treated with autologous BMMNCs followed by rigorous rehabilitation, 3 years after stroke.
Case Report
A 60 year old male patient with left sided hemiplegia due to ischemic stroke 3 years ago was evaluated. The ischemic episode started with giddiness and headache which was followed by tonic clonic movements and weakness of upper extremities and subsequently weakness of lower extremities. He developed right sided gaze palsy, slurred speech, difficulty passing urine followed by quadriplegia. He was immediately admitted to the hospital where he underwent elective intubation and was put on the ventilator. Magnetic resonance (MR) angiography of brain and neck showed complete occlusion of basilar artery with flow in terminal basilar artery and occlusion of both posterior cerebral arteries via posterior communicating artery and right vertebral artery occlusion. The flow void of the basilar artery was not seen which could suggest thrombosis. Magnetic Resonance Imaging (MRI) of brain revealed acute infarct involving right sided pons and few discrete foci in bilateral fronto-parietal white matter. He was admitted in the Intensive Care Unit (ICU) and treated with low molecular weight (LMW) heparin, anti-platelets, anti-hypertensives and other supportive medication. Tracheostomy was performed in response to sinus bradycardia and percutaneous endoscopic gastronomy (PEG) was performed for feeding. Persistent bradycardia was treated with metaproterenol sulphate thrice a day. Neurologic recovery was noted within one month, with residual right gaze palsy, spasticity in all four limbs and quadriparesis. Sensations in all the four limbs recovered, with control of bladder and bowel. Level of alertness had increased with good comprehension and cooperation in following commands. One month after acute ischemia he was discharged from the acute care hospital and continued regular rehabilitation at home. On discharge repeat MR angiography of brain and neck showed re-canalization of proximal basilar and intracranial portion of entire left vertebral arteries with persistent occlusion of mid basilar artery. Regular rehabilitation at home brought about significant improvement in the right lower extremity voluntary control, remission of motor and sensory loss on the right side and complete bowel and bladder control. In spite of regular rehabilitation under professional guidance, persistent motor deficits like increased spasticity and poor voluntary control of left upper and lower extremities and left hemiparesis, prompted him to explore newer treatment options.
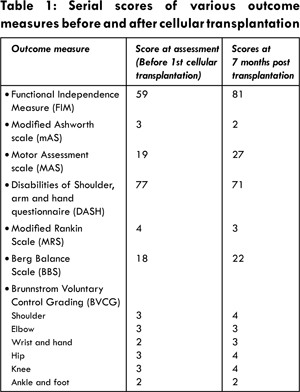
At assessment prior to cellular transplantation, patient was hypertonic with spasticity of Grade 3 on Modified Ashworth Scale (mAS) in the muscles of left upper limb and lower limb. Left upper limb exhibited flexor synergy pattern and left lower limb showed extensor synergy pattern. He had flexor spasms at night. Voluntary control of hip, knee, shoulder and elbow of left side of the body as measured on the Brunnstrom voluntary control grading (BVCG) was grade 3 and grade 2 for left wrist and ankle. Disabilities shoulder, arm and hand symptom questionnaire score was 77. Motor Assessment Scale (MAS) score was 19. There was no sensory or cognitive impairment. Modified Rankin Scale (MRS) score was 4. He had residual left facial palsy, speech was laborious and slurred. Tongue and lip movements were slow and indicated inflexibility. There was mild difficulty in swallowing solid food and puffing cheeks to blow air. Speech intelligibility (SI) score was 3. Ambulation was with stick in right hand and human assistance. Gait showed classical hemiplegic pattern. There was a left ‘foot drop’ noted clearly in the swing phase of gait, due to inability to selectively control the ankle dorsiflexors. Left hip-knee flexion during swing phase was reduced. Sitting and standing balance was poor. Berg balance score was 18. Functionally, he was dependent for almost all his activities of daily living (ADLs). Functional Independence Measure (FIM) score was 59 showing dependency in the areas of self-care like bathing, dressing upper and lower body, sphincter control, transfers, expression and social interaction.
MRI and Diffusion tensor imaging (DTI) with fibertracking through the pons showed thinning of the descending right corticospinal tract as compared to the contralateral side. Gliotic area was observed involving the right hemipons representing sequel of an old infarction. Rationale for cellular transplantation in this patient was based on Revised World Medical Association Helsinki Declaration for Ethical Principles for medical research involving human subjects [ 7]. A thorough pre-transplantation assessment was conducted including necessary investigations. Granulocyte Colony Stimulating Factor (GCSF) was administered 48 hours and 24 hours before the harvest and transplantation of BMMNCs to enhance the mobilization [ 8]. Bone marrow was aspirated by a standard procedure. Mononuclear cells were isolated by density gradient method. Viability and CD34+ count was performed. Intrathecal transplantation of the autologous bone marrow mononuclear cells was done by a standard lumbar puncture procedure. The procedure was identical to our previous reports and trials [ 3, 4]. He was monitored for any immediate in-hospital adverse events for one week post-transplantation and late adverse events for at least six months. This was followed by multidisciplinary rehabilitation including physiotherapy, occupational therapy, speech therapy and psychological intervention.
At the time of discharge from the hospital after stem cell therapy, patient showed improvement in pronunciation of words, increase in confidence level, and increase in range of motion at elbow. Because of reduced spasticity in left lower extremity from mAS grade 3 to mAS grade 2, he could walk independently for 2 floors at the time of discharge. Over a period of 7 months after stem cell therapy, many changes were noticed. Voluntary control of left shoulder and hand had improved [Table 1]. Movement of the left shoulder was possible through a greater range of motion and could open his left fist unlike prior to cellular therapy. Transfer activities like stand to sit and sit to stand were independent. Sitting down was more controlled. Static sitting and standing balance had improved. He could walk 15-20 steps independently with cane and no human assistance. Effort of speech had reduced. Lip and tongue movements were more flexible. Speech intelligibility score improved from 3 to 2. He was independent in performing most of the ADLs and there was a significant improvement in the FIM score. Maintaining self-hygiene was completely independent. Now he had good control of bowel and bladder and needed minimal assistance for transfer to commode. There was improvement in social interaction. Ambulation indoors was independent and outdoor ambulation was independent only with supervision. Stair climbing was independent with support of railing. All the outcome measures showed a significant change in the scores [Table 1].
Principle goal of management is to limit the morbidity and mortality associated with stroke. Current acute ischemic stroke management consists of prompt administration of intravenous tissue plasminogen activator and other thrombolytic and antithrombotic medication. The early administration of thrombolysis for the ischemic stroke leads to reduced morbidity at a chronic stage [ 9]. In the subacute and chronic stage rehabilitation is the focus of stroke management. Rehabilitative therapies make use of the plasticity of neural networks that are capable of reorganization in response to functional training stimuli [ 10]. In spite of advances in these management strategies, stroke is still the fourth leading cause of disability world over [ 11]. Stroke leads to 20% of the patients requiring to be institutionalized within 3 months and one third of the patients suffering from stroke suffer from permanent disability [ 12]. These residual deficits could be attributed to limited reparative and regenerative potential of the current management strategies.
Pathophysiological mechanisms of benefits observed post stem cell transplantation: Stem cell therapy has the potential to induce neuro-restoration essential for facilitating recovery of neurological function [ 5]. In various animal studies it has shown to reduce the infarct size and improve the functional and neurological recovery. Cells were administered to rats after ischemic stroke through intravenous, intra-arterial and intracarotid routes. The cells were injected within the acute stroke period ranging from 24 hours to 1 month [ 1, 2, 13, 14, 15]. The effects are therefore thought to be due to mechanisms other than actual neurogenesis from the transplanted cells. BMMNCs have been found to secret various neurotrophic factors and anti-inflammatory cytokines including interleukin-10, insulin-like growth factor-1, vascular endothelial growth factor, and stromal cell-derived factor-1 [ 5]. In addition to these neuroprotective mechanisms; paracrine mechanisms like angiogenesis, reduction in the cell apoptotic processes and cell death, stimulation and relocation of the existing local stem cells and reduction of the inflammatory processes that are triggered in response to the injury are believed to bring about the clinical outcomes noticed after BMMNCs transplantation [ 2, 5, 16, 17]. These mechanisms are believed to be more potent in bringing about the clinical changes than actual neuronal differentiation of the transplanted cells [ 5]. We postulate that the reparative processes are more effective in the penumbra and are exhibited as clinical improvements.
Human trials of autologous BMMNCs have also reported to be safe with no adverse effects [ 3, 4]. Intravenous and intra-arterial transplantation of BMMNCs in human trials has shown functional and neurological improvement. The recovery was also marked by the change of one unit or more in the scores of modified ranking scale [ 18- 20]. This case report highlights the effects of cellular transplantation at a chronic stage. The results were synonymous with the previous findings where we found a one point reduction in MRS scale 7 months post therapy. Patient showed clinically significant reduction in spasticity (MAS 3 to 2), improved voluntary control (BVCG 3 to 4) and reduction in synergy patterns. SI score reduced from 3 to 2. All the outcome measures showed an improvement post cellular transplantation. Stem cell transplantation facilitated functional improvement and reduction in spasticity. One may debate that the stem cells transplantation may have triggered the reparative body mechanisms through various paracrine mechanisms. The continued rehabilitation post stem cell therapy may have augmented the benefits of cellular therapy. This augmentation is presumed to be due to facilitation of neuroplasticity.
Conclusion
The clinical improvement observed in this case report is suggestive of potential benefits of cellular therapy in chronic ischemic stroke. This preliminary evidence suggests that trials should be initiated for further understanding of the efficacy of stem cell transplantation for the treatment of chronic stroke.
Research Highlights
- No major or minor adverse events were noted with autologous BMMNCs intrathecal transplantation in chronic ischemic stroke.
- Functional improvements were observed despite chronicity of the condition.
- Quality of life improved post cellular transplantation.
References
- Brenneman M, Sharma S, Harting M, Strong R, Cox CS Jr, Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010; 30(1):140-149.
- Savitz SI, Misra V, Kasam M, Juneja H, Cox CS Jr, Alderman S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70(1):59-69.
- Sharma A, Gokulchandran N, Sane H, Badhe P, Kulkarni P, Lohia M, et al. Detailed analysis of the clinical effects of cell therapy for thoracolumbar spinal cord injury: an original study. Journal of Neurorestoratology. 2013;1:13-22.
- Sharma A, Gokulchandran N, Sane H, Nagrajan A, Paranjape A, Kulkarni P, et al. Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem Cells Int. 2013;2013:623875.
- Gnecchi, Massimiliano, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circulation Research. 103;11(2008):1204-1219.
- Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213-228.
- Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. British Journal of Clinical Pharmacology. 2004;57(6):695-713.
- Haas R, Murea S. The role of granulocyte colony-stimulating factor in mobilization and transplantation of peripheral blood progenitor and stem cells. Cytokines Mol Ther. 1995; 1(4):249-270.
- Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart association/American Stroke Association. Stroke. 2013;44(3):870-947.
- Johansson BB. Current trends in stroke rehabilitation. A review with focus on brain plasticity. Acta Neurol Scand. 2011;123(3):147-159.
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6(2):182-187.
- Steinwachs DM, Collins-Nakai RL, Cohn LH, Garson A Jr, Wolk MJ. The future of cardiology: utilization and costs of care. J Am Coll Cardiol. 2000;35(5 Suppl B):91B-98B.
- Minnerup J, Seeger FH, Kuhnert K, Diederich K, Schilling M, Dimmeler S, et al. Intracarotid administration of human bone marrow mononuclear cells in rat photothrombotic ischemia. Exp Transl Stroke Med. 2010;2(1):3.
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330-338.
- Kamiya N, Ueda M, Igarashi H, Nishiyama Y, Suda S, Inaba T, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;12,83(11-12):433-437.
- Lindoso RS, Araujo DS, Adão-Novaes J, Mariante RM, Verdoorn KS, Fragel-Madeira L, et al. Paracrine interaction between bone marrow-derived stem cells and renal epithelial cells. Cell Physiol Biochem. 2011;28(2):267-278.
- Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56(7):593-607.
- Prasad K, Mohanty S, Bhatia R, Srivastava MV, Garg A, Srivastava A, et al. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: a pilot study. Indian J Med Res. 2012;136(2):221-228.
- Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Piñero P, Espigado I,Garcia-Solis D, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43(8):2242-2244.
- Friedrich MA, Martins MP, Araújo MD, Klamt C, Vedolin L, Garicochea B, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012; 21 Suppl 1:S13-21.
|