Introduction
Topiramate is an oral antiepileptic drug (AED) used for partial onset, generalized primary tonic-clonic seizures [
1] and migraine prophylaxis [
2]. It has a novel chemical structure derived from D-fructose that has several mechanisms of actions. It blocks voltage-sensitive sodium channels, enhances the activity of GABA, an inhibitory neurotransmitter, and blocks the action of glutamate, an excitatory neurotransmitter [
3]. Topiramate-induced bradycardia is a rare condition of unknown etiology and mechanism. Conventional anti-epileptic drugs e.g carbamezepine [
4], phenytoin [
5,
6] have been known to cause bradycardia.
Case Report
A 26 year old house wife presented with complaints of light-headedness and palpitations since the last 4 days. She denied any syncopal episodes, shortness of breath, chest pain and vertigo. There was no history of similar episodes in the past. She however mentioned that she gets tired easily after doing household work like running a vacuum cleaner and going upstairs. She had a background history of migraine and hypothyroidism, but no history of heart disease in the past. Her current medications were levothyroxine 50 micrograms daily and topiramate 25 mg twice a day for migraine prophylaxis. She denied using any over the counter medications or substance of abuse. She had been started on topiramate two weeks ago with a dose of 25 mg once a day in first week and then titrated up to 25 mg twice a day in the week which she presented with the symptoms. She had no family history of heart disease or sudden cardiac death. She was a non-smoker and occasionally had alcohol.
On examination she weighed 82 kilograms and was 5 feet and 5 inches tall. She had a slow pulse at a rate of 44 beats per minute on examination. The blood pressure was 110/60 mmHg with oxygen saturations of 99% on room air and was apyrexial. The heart and breath sounds were normal and there were no added sounds. The abdominal and neurological examinations were also unremarkable. She had an electrocardiogram done which showed sinus bradycardia at a rate of 46 beats per minute [Fig.1]. The ST segment, PR interval or QT intervals were normal. We carried out a lying and standing blood pressure which did not show any postural drop. The chest X-ray was also un-remarkable.
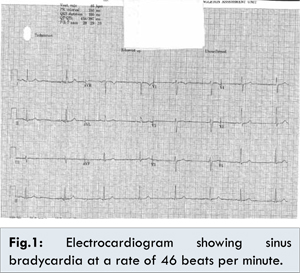
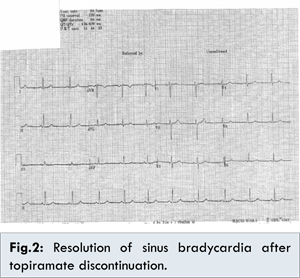
Based on her past medical history of hypothyroidism, it was considered that her bradycardia was possibly secondary to under-treated hypothyroidism but on evaluation of her thyroid status the thyroid function tests were also normal. Similarly the blood tests failed to reveal electrolyte imbalance (Normal serum sodium, potassium, calcium, chloride, magnesium, phosphate) as a possible cause of bradycardia. We offered the patient ECG telemetry on the ward which she accepted and on the telemetry, sinus bradycardia was the only rhythm which was noted. An echocardiogram was done which was reported as normal left ventricular function and no evidence of structural heart disease. This left us with a dilemma as to the cause of the bradycardia. We then thought of reviewing her history and consequently stopped topiramate to see if this would help. Remarkably the sinus bradycardia resolved on approximately 30 hours after discontinuation of topiramate [Fig.2]. Her symptoms resolved with the resolution of the bradycardia. She was subsequently started on amitriptyline for migraine prophylaxis. On follow up after 2 weeks, no bradycardia was demonstrated.
Sinus bradycardia can be defined as a sinus rhythm with a resting heart rate of 60 beats per minute or less. However, few patients actually become symptomatic until their heart rate drops to less than 50 beats per minute [
7]. The pathophysiology of sinus bradycardia is dependent on the underlying cause. Common causes of bradycardia include: aging, myocardial infarction, hypertension, congenital heart disease, myocarditis, hypothyroidism, electrolyte imbalance, sleep apnea and medication [
8]. While investigating a patient with bradycardia it is important to elicit the past medical history of heart disease and especially a family history of sudden cardiac death. Investigations would involve checking for electrolytes i.e calcium, magnesium, potassium and glucose, thyroid function tests and a toxicology screen if possible [
9].
The topiramate induced symptomatic bradycardia was assessed as “probable” based on the Naranjo Adverse Drug Reaction scale [
10,
11]. [Table 1]. The possible mechanism of bradycardia is still unknown but could be secondary to several cellular targets have been proposed to be relevant to the therapeutic activity of topiramate [
12]. These include voltage-gated sodium channels and high-voltage-activated calcium channels and although the voltage-gated Na+, Ca2+ and K+ channels expressed in the heart are often distinct but the they are closely homologous to those expressed in the brain [
13].
The limitations of this report include the absence of plasma topiramate level measurement and electrophysiological studies; however, these examinations were not practical because of the patient’s dramatic improvement after stopping topiramate.
Conclusion
Drug induced bradycardia is a category that all health care professionals should remember in investigations for bradycardia. Although bradycardia is a rare adverse reaction to anti-epileptics, this finding may alert neurologists and physicians to this anti-epileptic drug side effect and consideration for routine pulse monitoring and possibly electrocardiographic monitoring while up titrating the dose of topiramate in those who present with symptoms of bradycardia. Further studies are needed to understand the role of topiramate in bringing about symptomatic bradycardia.
References
- Clinicalkey.com. ClinicalKey. 2016. Available at: https://www.clinicalkey.com/#!/content/drug_monograph/6-s2.0-821. Accessed February 8, 2016.
- Linde M, Mulleners W, Chronicle E, McCrory D. Topiramate for the prophylaxis of episodic migraine in adults. The Cochrane Database of Systematic Reviews-Issue 7 2013. Journal of Evidence Based Medicine. 2013;6(3):197-198.
- Porter RJ, Dhir A, Macdonald RL, Rogawski MA. Mechanisms of action of antiseizure drugs. Handb Clin Neurol. 2012;108:663-681.
- Kaul S, Meena A, Murthy J. Carbamazepine induced bradycardia. Neurol India. 2000;48:403.
- York R, Coleridge S. Cardiopulmonary arrest following intravenous phenytoin loading. The American Journal of Emergency Medicine. 1988;6(3):255-259.
- Earnest M. Complications of intravenous phenytoin for acute treatment of seizures. Recommendations for usage. The Journal of the American Medical Association. 1983; 249(6):762-765.
- Livingston M. Sinus Bradycardia: Background, Pathophysiology, Epidemiology. Emedicinemedscapecom. 2016. Available at: http://emedicine.medscape.com/article/760220-overview. Accessed February 8, 2016.
- Ganz L. Sinus bradycardia. Uptodatecom. 2015. Available at: http://www.uptodate.com/contents/sinus-bradycardia. Accessed February 8, 2016.
- Livingstone M. Sinus Bradycardia Workup: Laboratory Studies, Imaging Studies. Emedicinemedscapecom. 2016. Available at: http://emedicine.medscape.com/article/760220-workup. Accessed February 8, 2016.
- Naranjo C, Shear N, Lanctôt K. Advances in the Diagnosis of Adverse Drug Reactions. The Journal of Clinical Pharmacology. 1992;32(10):897-904.
- Naranjo C, Busto U, Sellers E, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
- Porter RJ, Dhir A, Macdonald RL, Rogawski MA. Mechanisms of action of antiseizure drugs. Handb Clin Neurol. 2012;108:663-681.
- Meldrum B, Rogawski M. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4(1):18-61.