Introduction
Bezold’s abscess is complication of otitis media, which was first reported in 1881 and occurs when a purulent otomastoiditis erodes the mastoid tip [
1]. Sub-periosteal abscesses, that arise from the erosion of the outer surface of the mastoid process, are more common than Bezold’s ones, in which the pus can run along the fascial surfaces of digastric and sternocleidomastoid muscles, with potential devastating consequences if the infection descend towards perivisceral spaces, larynx or mediastinum. The cholesteatoma increases risk of complications because of its aggressiveness and its intrinsic tendency to recur [
2-
4]. We report the case of a patient who presented Bezold’s abscess as first sign of a recurrent cholesteatoma, 38 years after a canal wall down tympanoplasty.
Case Report
A 64-year-old man presented to our department in July 2015 complaining of history of right-sided otalgia, otorrhea, and postauricular tender swelling with local hyperemia. His past medical history was significant for right tympanoplasty with mastoidectomy for treatment of cholesteatoma: the patient’s right ear had been operated using the canal wall down technique in 1977, not performing follow up for the last 15 years. He arrived to our attention presenting signs of right oto-mastoiditis with omolateral facial nerve palsy (III grade according to House-Brackmann scale) and a swelling of the right upper neck. Blood tests revealed no leukocytosis and C-reactive protein (CRP) level was above normal [39.00 mg/L (normal: 0-6 mg/L)]. He was admitted by us for further management. Micro-otoscopic examination revealed purulent discharge from the right radical tympanoplasty cavity. Electromyography (EMG) showed spontaneous voluntary muscular activity in mouth and eye orbicular right muscles. Computed tomographic (CT) scan demonstrated a dis-homogeneous density area of 2.5x3 cm in diameter, with presence of air, filling the region from the mastoid process through the sulcus for the sigmoid sinus with radiologic evidence of extensive bony destruction of the temporal bone [Fig.1,2,3,4]. A revision of radical mastoidectomy and cervicotomy with extensive abscess drainage under general anesthesia was performed. Intra-operatively the mastoid cavity appeared filled with choleasteatomatous tissue, with complete erosion of facial nerve canal. The dura of the posterior cranial fossa was exposed with signs of pachimeningitis. Lateral sinus disappeared. There was bony erosion of the mastoid tip around the digastrics ridge which created a communication for the upper neck abscess. The temporal bone was also extensively eroded, creating a large cavity deeply extended to the jugular bulb. The abscess was evacuated, the affected region was irrigated, and a drain was positioned. The cholesteatoma debris was removed, and meatoplasty was completed to open the path widely between the mastoid and the external auditory canal. Staphylococcus epidermidis was cultured from the microbiologic samples taken intraoperatively. The drain was removed on the third postoperative day. Intravenous administration of antibiotics was continued after surgery. The CT performed a month later showed resolution of the infection. Revision of right mastoidectomy conducted a year later demonstrated no recurrence of cholesteatoma.
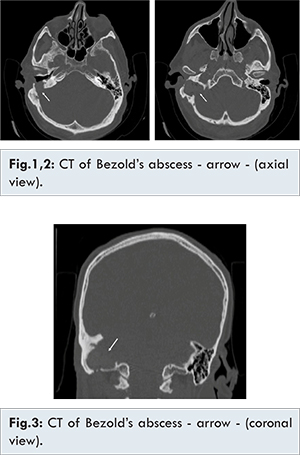
It’s well known that cholesteatoma has tendency for recurrence and bone erosion [
5], leading to complications that rarely include Bezold’s abscess, whose pathogenesis has been attributed to the degree of mastoid’s pneumatisation: in front of a poor ventilation of mastoid cells, the hard and thick bony walls prevent the spreading of the disease [
6]. In our case, massive cholesteatoma in the mastoid could result in the erosion of the mastoid bone, especially as prior mastoidectomy had created a pathway towards mastoid apex, enabling the infection to spread down through the fascial planes of digastrics and sternocleidomastoid muscles. Usually chronic suppurative otitis media underlies Bezold’s abscess, with or without cholesteatoma [
7]. Since the widespread use of antibiotics, the incidence of acute otitis media causing a complicated otitis media has declined, and clearly a chronic inflammation is at the basis of most otitic suppurative complications [
4,
7]. In 53-78.5% of patients with extracranial and intracranial complications, cholesteatoma has been found at surgical exploration [
2,
3,
4], and several studies reported Bezold’s abscess arising from cholesteatoma [
5,
7-
13]. However, only two papers describe cholesteatoma recurrence with Bezold’s abscess presented many years after first surgery [
7,
13], as in our case report.
Literature describes various reports quoting the incidence of post-operative drainage after open cavity mastoidectomy. Many authors have demonstrated a success rate of 70-95% [
14,
15]. The findings responsible for failure of this type of surgery could include nonfunctional meatoplasty and/or presence of inflammatory granulation tissue which occludes drainage path from the mastoid to the external auditory meatus. If this granulation tissue occupies the antrus, it could hide and force an underlying cholesteatoma. CT scan of temporal bone and neck is the main radiological tool for the diagnosis of Bezold’s abscess, but enhanced temporal bone T1, T2 and diffusion-weighted MRI is binding to rule out cholesteatoma of middle ear [
13]. Wide spectrum antibiotics and surgical exploration are required, with drainage of both mastoid and deep neck infection to eradicate the diseased mastoid and to prevent sequel [
7]. As regards microbiologic samples, Streptococcus spp. is the most commonly isolated organism, followed by Proteus mirabilis, Staphylococcus aureus and Proteus vulgaris [
5].
Unexpected serious complications developed from otitis media can be secondary to inadequate dosing or timing of antibiotic therapy, presence of resistant bacterial strains and discovery of underling cholesteatoma, in particular when there is a recurrence of the disease or a personal history of surgery of the middle ear, with consequent changes in the anatomy of the site.
Conclusion
Our case demonstrates that serious suppurative and rare complications as Bezold’s abscess can be caused by the recurrence of cholesteatoma, even many years after primary surgery. Surgical approach and wide spectrum antibiotics are mandatory to eradicate the infection and prevent consequent complications.
References
- Bezold F. Ein neuer Weg für die Ausbreitung eitriger Entzündung aus den Raumen des Mittelohrs auf die Nachbarschaft. Deutsche medizinische. Wochenschrift. 1881;28:381-384.
- Spiegel JH, Lustig LR, Lee KC, Murr AH, Schindler RA. Contemporary presentation and management of a spectrum of mastoid abscesses. Laryngoscope. 1998;108(6):822-828.
- Osma U, Cureoglu S, Hosoglu S. The complications of chronic otitis media: report of 93 cases. J Laryngol Otol. 2000;114:97-100.
- Kangsanarak J, Fooanant S, Ruckphaopunt K, Navacharoen N, Teotrakul S. Extracranial and intracranial complications of suppurative otitis media. Report of 102 cases. J Laryngol Otol. 1993;107:999-1004.
- Lazim NM, Abdullah A. An extensive cholesteatoma with Bezold’s abscess. Inter J Clin Med. 2011;2:292-294.
- McMullan B. Bezold’s abscess: a serious complication of otitis media. J Paed and Child Health. 2009;45(10):616-618.
- Uchida Y, Ueda H, Nakashima T. Bezold’s abscess arising with recurrent cholesteatoma 20 years after the first surgery: with a review of the 18 cases published in Japan since 1960. Auris Nasus Larynx. 2002;29:375-378.
- Aberbach A, Joachims HZ, Goldsher M. A neck mass as an unusual complication of aural cholesteatoma. Ear Nose Throat J. 1998;67(10):780-781.
- Hughes GB, Damiani KA, Kinney SE, Levine HL. Aural cholesteatoma presenting as a large neck mass. Otolaryngol Head Neck Surg. 1980;88(1):34-36.
- Topaloglu I, Uguz MZ, Ardiç FN. Giant cholesteatoma presenting as a postauricolar mass. Otolaryngol Head Neck Surg. 1997;116(6.1):678-679.
- Furukawa K, Arai E, Kobayashi T, Takasaka T. A case of Bezold’s abscess associated with cholesteatoma. Nihon Jibiinkoka Gakkai Kaiho. 1992;95(12):1901-1905.
- Janardhan N, Nara J, Peram I, Palukuri S, Chinta A, Satna K. Congenital cholesteatoma of temporal bone with Bezold’s abscess: case report. Indian J Otolaryngol Head Neck Surg. 2012;64(1):97-99.
- Lionello M, Manara R, Lora L, Mylonakis I, Fasanaro E, La Torre FB, Ottaviano G, Staffieri A, MarcheseRagona R. Case report of cholestetaoma recurrence with Bezold’s abscess presenting as a deep neck infection. B-ENT. 2013;9:255-258.
- Prasanna Kumar S, Ravikumar A, Somu L. Modified radical mastoidectomy: a relook at the surgical pitfalls. Indian J Otolaryngol Head Neck Surg. 2013;65:548-552.
- Li S, Meng J, Zhang F, Li X, Qin Z. Revision surgery for canal wall down mastoidectomy: intra-operative findings and results. Acta Otolaryngol. 2016;136(1):18-22.