6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff6422190000003403000001000a00
6go6ckt5b5idvals|755
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Fibroadenoma is the most common breast tumor both clinically and pathologically in adolescent and young women. Cystic changes, usually measuring between 1-10 mm, may occur within these benign fibroepithelial tumors. Fibroadenomas that consist of cysts greater than 3 mm, sclerosing adenosis, epithelial calcifications, and/or papillary apocrine metaplasia are considered as complex fibroadenoma. It is well known that the relative risk of developing breast carcinoma in patients with complex fibroadenoma is increased, compared to women with non-complex fibroadenoma. Predominant cystic degeneration of the tumor that grossly constitutes most of the tumor, so called “cystic fibroadenoma,” is rare. Herein, we present a rare case of cystic fibroadenoma of the breast in a 32-year-old female.
Case Report
A 32 years old female presented with the complaint of lump in left breast for one year. Clinical examination revealed a non-tender, mobile, well circumscribed cystic lesion in the lower inner quadrant of the left breast. Fine needle aspiration (FNA) showed bimodal population of ductal epithelial cells admixed with darkly stained spindle shaped myoepithelial cells in a thick proteinaceous background suggesting fibroadenoma with cystic changes [Fig.1].
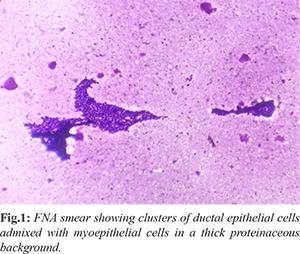
Lumpectomy was done and specimen was sent for histopathological examination. Gross examination revealed a well circumscribed lesion measuring approximately 6×4×2 cm. There was marked cystic degeneration with a jelly-like dense homogeneous, dark yellow material in its lumen [Fig.2]. Histopathologic examination of the lesion demonstrated multiple cysts filled with dense secretory material. Surrounding breast parenchyma consisted of fibroadenomatoid nodules with elongated compressed ducts and stromal proliferation [Fig.3]. The lesion was diagnosed as fibroadenoma of the breast with marked cystic degeneration, and because the largest dimension of the cyst was greater than 3 mm, it was considered as a complex fibroadenoma.
Discussion
A benign biphasic tumor fibroadenoma occurs most frequently in women of childbearing age, especially those under 30 years. Usually considered a neoplasm, some believe fibroadenoma results from hyperplasia of normal lobular components rather than being a true neoplasm. Fibroadenoma presents as a painless, solitary, firm, slowly growing (upto 3 cm), mobile, well defined nodule. Less frequently it may occur as multiple nodules arising synchronously or asynchronously in the same or in both breasts and may grow very large (upto 20 cm) mainly when it occurs in adolescents. Such lesions may be called “giant” fibroadenomas.
The cut surface is solid, firm, bulging, greyish in color, with a slightly lobulated pattern and slit like spaces. Variations depend on the amount of hyalinization and myxoid change in the stromal component. Calcification of sclerotic lesions is common. Over time, this tumor may undergo some degenerative changes, such as myxoid degeneration, metaplastic changes including smooth muscle (myoid) metaplasia, adipose differentiation, rarely osteochondroid metaplasia, or infarction [
1-
3]. Fibroademoma having cysts greater than 3 mm, or associated sclerosing adenosis, epithelial calcifications, or papillary apocrine metaplasia is considered as complex fibroadenoma. Predominant cystic degeneration of this tumor is very rare [
1]. Nevertheless, cystic fibroadenoma, that is thought to arise from ectopic mammary glands have been reported in other organs as well, such as vulva [
4] and prostate [
5].
The admixture of stromal and epithelial proliferation gives rise to two distinct growth patterns of no clinical significance. The peri-canalicular pattern is the result of proliferation of stromal cells around ducts in a circumferential fashion; this pattern is observed most frequently during the second and third decades of life. The intra-canalicular pattern is due to compression of the ducts into clefts by the proliferating stromal cells. The stromal component may sometimes exhibit focal or diffuse hyper-cellularity (especially in women less than 20 years of age), atypical bizarre multi-nucleated giant cells, extensive myxoid changes or hyalinization with dystrophic calcification. The epithelial component can show a wide spectrum of typical hyperplasia, mainly in adolescents, and metaplastic changes such as apocrine or squamous metaplasia may be seen. Foci of fibrocystic change, sclerosing adenosis and even extensive myoepithelial proliferation can also occur in fibroadenoma.
Mammography reveals a homogeneous, round or oval, circumscribed mass in fibroadenoma, but fails to distinguish whether a mass is solid or cystic. Ultrasound examination is investigation of choice for characterizing cystic breast masses as predominantly cystic masses with solid components, or predominantly solid masses with cystic components [
6]. Predominantly cystic lesions include simple cysts, traumatic and postoperative fluid collections, abscess, galactocele, cystic papilloma and cystic papillary carcinoma. Complex breast cysts, that are defined as cysts with thick walls, thick septa, intracystic masses, or other discrete solid components, include both benign lesions, such as fibrocystic changes, intraductal or intracystic papilloma without atypia, fibroadenoma; atypical or high risk lesions such as atypical ductal hyperplasia, atypical papilloma; and malignant lesions, such as ductal carcinoma in situ, invasive ductal and invasive lobular carcinoma [
6,
7]. Clinical, imaging, and pathologic correlation is significant in these lesions for appropriate management of the patient.
Various studies have reported that fibroadenomas do not have increased risk for developing breast carcinoma, unless the tumor or the surrounding breast parenchyma shows proliferative changes, or the patient has a family history of breast cancer [
2,
8]. Dupont et al. have reported that the relative risk of developing breast carcinoma was 3.1 for women who had complex fibroadenoma, and that the risk had increased to 3.7 if the patient had complex fibroadenoma and a family history of breast carcinoma [
2].
Conclusion
Pathologic examination of the lesion is usually necessary in order to highlight the nature of the lesion. Cystic fibroadenoma, although rarely seen, should be considered in the differential diagnosis of cystic lesions of the breast that includes cystic papilloma and fibrocystic changes.
Contributors: VM: manuscript writing; PR, AO: manuscript editing, literature search; TA: manuscript editing and critical inputs into the manuscript. VM will act as guarantor. All authors approved the final version of the manuscript.
Funding: None; Competing interests: None stated.
References
- Rosen PP. Fibroepithelial neoplasms. In: Rosen PP (ed): Rosen’s Breast Pathology. 3rd ed., Philadelphia, Lippincott Williams & Wilkins, 2009;187-201.
- Dupont WD, Page DL, Parl FF, Vnencak-Jones CL, Plummer WD Jr, Rados MS, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10-15.
- Pinder SE, Mulligan AM, O’Malley FP. Fibroepithelial lesions, including fibroadenoma and phyllodes tumor. In: O’Malley FP, Pinder SE, Goldblum JR (eds): Breast Pathology. 1st ed., Philadelphia, Churchill Livingstone Elsevier: 2006;109-115.
- Menet E, Wager I, Babin M, Magnin G, Babin P. Multiple vulvar cystic and papillary fibroadenomas. J Gynecol Obstet Biol Reprod. 1999;28:830-832.
- Gerridzen RG, McDonald MW, Mai KT. An unusual pelvic mass: cystic fibroadenoma of the prostate. Can J Urol. 1995;2:172-174.
- Cardenosa G. Cysts, cystic lesions, and papillary lesions. Ultrasound Clin. 2007;1:617-629.
- Doshi DJ, March DE, Crisi GM, Coughlin BF. Complex cystic breast masses: diagnostic approach and imaging-pathologic correlation. Radiographics. 2007;27:53-64.
- Hutchinson WB, Thomas DB, Hamlin WB, Roth GJ, Peterson AV, Williams B. Risk of breast cancer in women with benign breast disease. J Natl Cancer Inst. 1980;65:13-20.