|
Murugaiyan Nagarajan, Ramesh Banu, Karuvandan Naveen, Athisyaraj Patrick Joshua Valavadi Narayanasamy Cancer Centre, G. Kuppuswamy Naidu Memorial Hospital, Coimbatore-641037, Tamil Nadu, India.
Corresponding Author:
Dr. M. Nagarajan Email: mnr81@yahoo.com
Abstract
Background: In recent days with the advent of advanced diagnostic and treatment modalities, the survival of cancer patients has improved but there is rise in multiple primary cancers, the most common are double primary malignancies. The present analysis was to report our observed frequency of double malignancies among the cancer patients. Material and Methods: This is a retrospective study among the patients diagnosed with histologically proven double malignancies (synchronous and metachronous) from January 2009 to July 2014 from our hospital based cancer registry. Results: A total of 104 patients with double malignancies either synchronous or metachronous were identified during our study period. 40 patients (38%) had synchronous and 64 patients (62%) had metachronous malignancies. The most common site for first malignancy was head and neck and female genital tract which constituted about 29% each followed by breast, gastrointestinal tract. Most common second site of malignancy was gastrointestinal tract followed by breast and brain. In metachronous malignancy mean time for diagnosing second malignancy was 6.67 years. The 5 year overall survival was 61% in metachronous malignancy. Median overall survival was 6.21 years. In synchronous malignancy median overall survival was 51 days. Only 2 patients survived more than 2 years. Overall metachronous malignancy had better prognosis than synchronous malignancy. Conclusion: It is imperative that patients with a primary tumor should be thoroughly, closely and regularly followed. Genetic counseling, risk estimation, cancer screening and chemoprevention must be emphasized.
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffa86a270000007107000001000800 6go6ckt5b5idvals|920 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Malignancies that are occurring in two different organs at the same time is rare. The incidence is reported to be 0.52% to 11.7% from various studies [ 1- 3]. The double primary malignancies which was thought to be rare occurrence, now days seems to occur with an increased probability of being diagnosed more frequently than earlier due to increased awareness, improved diagnostic facilities and cancer treatment. In different regions, the incidence, characteristics and survival rates associated with these type of cancers have been found to be diverse and they also pose a challenge to the treating physician and also have an impact on the morbidity and mortality of the patient. Case Series
This is a retrospective analysis in a tertiary care centre from our hospital based cancer registry. We analyzed patient data retrospectively from January 2009 to July 2014 from the hospital registry. Patients were diagnosed with double primary malignant tumor either synchronous or metachronous as per the criteria proposed by Warren and Gates as follows [ 4, 5]: each of the tumor must be histologically confirmed malignant tumor, must be geographically distinct, lesions separated by normal mucosa, and probability of one being metastasis of other must be excluded. Synchronous malignancies refers to those where the second malignancy was diagnosed within six month of diagnosing primary cancer and metachronous is diagnosed after six months of diagnosing primary malignancy. Patients without biopsy were excluded from the study. The cases were analyzed for the type of malignancies, histology of the tumor, age, gender of the patient, time interval between first and second tumor, follow up of these patients, date of last visit and their status were obtained. The overall survival (OS) was calculated from the date of tumor diagnosis to the date of death or last date of follow up.
Results
During our study period over four years 12,915 patients were screened for enrollment between January 2009 to July 2014. Out of this, 104 patients with double primary malignancies were enrolled based on inclusion and exclusion criteria. 40 patients (38%) had synchronous and 64 patients (62%) had metachronous malignancies. The detailed demographic profile of synchronous and metachronous malignancies are mentioned in Table 1. In synchronous malignancy about 50% presented in stage I, 11% in stage II, 33% in stage III, 6% had stage IV presentation at first site, and at the second site 37% in stage I, 26% in stage II, 32% in stage III, 5% had stage IV. In metachronous malignancy 28% presented in stage I, 35% in stage II , 30% in stage III, 7% had stage IV presentation at first site, and at the second site 33% had stage I, 17% in stage II, 30% in stage III, 20% had stage IV. The frequent pathology types that were observed are adenocarcinomas (47%), squamous carcinomas (35%) and sarcomas (5%) and others (13%).
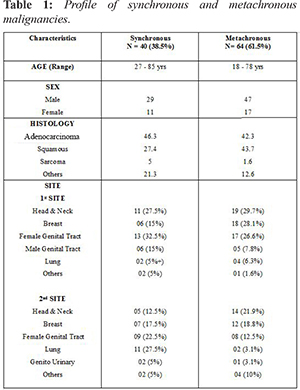
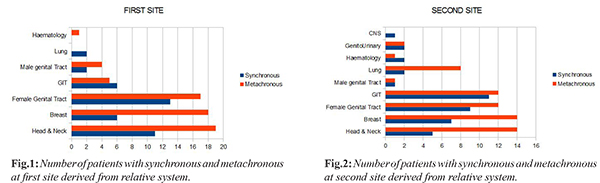
In our analysis, overall most common site of first malignancy was head & neck and female genital tract (29% each) while gastrointestinal tract (GIT) (22%) was most site of second malignancy [Fig.1,2]. In synchronous malignancy, most common site first diagnosed was female genital tract (33%) while the most common second malignancy site was GIT (27.5%). In metachronous malignancy, mean time interval between the time of diagnosing first malignancy and second malignancy was 6.67 years. In metachronous malignancy, most common first and second site of diagnosis was head & neck (30% and 22% respectively), hematological malignancy (1%) & neck and breast (22% each) followed by female genital tract & GIT (19% each), lung (13%) and other sites in 5%.
47.5% of patients with synchronous malignancy had history of substance abuse. In this subset 36.8% had history of smoking, 26.3% had history of alcohol abuse, 31.6% had beetel chewing habit and 5.2% had history of tobacco chewing. In metachronous malignancy, 29.7% had history of substance abuse. 42.1% had history of smoking, 31.6% had betel chewing habit and 26.3% had history of alcohol abuse. 12.5% (n=5) of patients with synchronous malignancy had family history of malignancy but only two patients had significant correlation. In metachronous malignancy, 6.2% (n=4) of patients had positive history of malignancy. In synchronous malignancy about 25% of patients underwent surgery, 32.5% of patients received multimodality treatment and 15% patients did not accept any treatment for the first primary cancer. For the second site of malignancy, 25% of the patients were treated with surgery alone, 17.5% of patients with chemotherapy alone and 20% with multimodality. In metachronous malignancy for the first site 7.8% of the patients were treated with surgery alone, 7.8% with radiotherapy alone and 1.6% with chemotherapy alone and 82.8% with multimodality treatment. For the second site 12.5% of the patients were treated with surgery alone, 12.5% with chemotherapy alone, 56.2% with multimodality treatment and 18.8% of patients did not undergo any form of treatment. In synchronous malignancy only two patients survived beyond two years. Median survival was 51 days. Median overall survival was 6.21 years in metachronous malignancy. The 5 year over all survival (OS) was 61%, 10 year survival was 23% and 15 year OS was 13% from the time of diagnosing first malignancy. Four patients with metachronous malignancy had OS more than 20 years.
Discussion
The incidence of multiple malignancies has not been rare. However in recent days we are able to come across increased number of multiple primary malignancies due to increased survival rate of patients due to improved diagnostic facilities and multimodality treatment. In our study period of four years 104 cases were diagnosed to have double malignancies. There is a preponderance for females to develop multiple malignancies [ 2, 9]. There is no exact mechanisms for the development of multiple primary malignancies but the possible causes could be due to the genetic susceptibility, immune system of patients, and exposure to carcinogens including chemotherapeutic agents and radiation used in treatment for many form of cancer. It is estimated that 5% of all cancer patients develop further independent cancers [ 6- 8]. Another important factor in the development of double malignancies is field cancerisation [ 10, 11]. Patients with head and neck cancer have 36% cumulative life time risk of developing second cancer. This was attributed to field carcinogenesis related to exposure to common risk factors like tobacco chewing, smoking and alcohol consumption [ 12]. The term field cancerisation was coined by Slaughter et al. in 1953, large areas of aero-digestive tract when continuously exposed to carcinogens can lead to alteration in the mucosal epithelium. Multifocal carcinomas can occur in this pre-conditioned epithelium due to random mutations. This theory was widely accepted and it can explain the occurrence of synchronous and metachronous malignancies in head and neck cancers. In our study out of nineteen patients treated for head & neck cancers nine developed metachronous malignancies in the same region. Another important contributing factor is genetic. Inherited cancer predisposition is a genetic condition that confers a higher likelihood of developing cancer, compared with the level of risk in the general population [ 13]. Inherited mutations can function in a dominant or recessive fashion, can confer different degrees of penetrance, and cause early or late-onset disease, leading to marked variations in disease presentation within the cancer population [ 14]. DNA-based genetic testing represents a crucial step in the identification of people with a high life-time risk of cancer, and for whom genetic counseling, screening and prevention may greatly improve either the chance of avoiding the onset of cancer, or the outcome of the disease [ 15]. When in a patient two active malignancies are diagnosed at the same time, the challenge is to find an anticancer therapy strategy that covers both cancer types without increased toxicity or relevant pharmacological interactions and without negative impact on the overall outcome [ 16]. In our study majority of cases in both synchronous and metachronous tumors received multimodality treatment that included chemotherapy, radiotherapy, hormonal treatment in addition to surgery. Survival in the synchronous group, having two malignancies early may correlate with the decreased survival rate. Our study showed a median survival of 51 days with longest median survival time in metachronous group was 6.21 years. According to the literature review it may be due to the fact that the first malignancy was diagnosed and treated earlier age thus allowing for longer lag time before the development of the second primary malignancy and thus improved overall survival from the time of first malignancy [ 17]. In general metachronous malignancies have better prognosis than synchronous as in our study [ 18, 19]. The possibility of multiple primary malignancies existence should always be considered during pre-treatment evaluation. There are some evidences that screening could improve outcomes among patients who might develop second malignancies [ 20].
Conclusion
It is imperative that patients with a primary tumor should be thoroughly, closely and regularly followed. Genetic counseling, risk estimation, cancer screening and chemoprevention must be emphasized.
References - Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: Case report and a comprehensive review of the literature. Am J Clin Oncol. 2003;26:79-83.
- Liu FS, Qin DX, Wang QL. The clinical pathology analysis of 172 cases of multiple primary malignant tumors. Zhonghua Zhong Liu Za Zhi. 1979;1:113.
- Li W, Zhan Y, Li G. Double cancers: a clinical analysis of 156 cases. Zhonghua Zhong Liu Za Zhi. 1996;18:296-298.
- Bagri PK, Singh D, Singhal MK, Singh G, Mathur G, Jalkar SL et al. Double primary malignancies: A clinical and pathological analysis report from a regional cancer institute in India. Iranian Journal of Cancer prevention. 2014;7(2):66.
- Warren S, Gates O. Multiple primary malignant tumors: A survey of the literature and statistical study. Am J Cance. 1932:16:1358-1414.
- Curtis RE, Boice JJ, Kleinerman RA, Flannery JT, Fraumeni JJ. Summary: multiple primary cancers in Connecticut, 1935-1982. National Cancer Institute Monograph. 1985;68:219-242.
- Storm HH, Jensen OM, Ewertz M, Lynge E, Olsen JH, Schou G, et al. Summary: multiple primary cancers in Denmark, 1943-80. National Cancer Institute monograph. 1985;68:411-430.
- Kobayashi Y, Arimoto H, Watanabe S. Occurrence of multiple primary cancer at the National Cancer Center Hospital, 1962-1989. Japanese Journal of Clinical Oncology. 1991;21(3):233-251.
- Liu Z, Liu C, Guo W, Li S, Bai O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015;10(5):e0125754.
- Noh SK, Yoon JY, Ryoo UN, Choi CH, Sung CO, Kim TJ, et al. A case report of quadruple cancer in a single patient including the breast, rectum, ovary, and endometrium. J Gynecol Oncol. 2008;19:265-269.
- Kim SH, Kim HJ, Lee JI, Lee YS, Kang WK, Park JK, et al. Multiple primary cancers including colorectal cancer. J Korean Soc Coloproctol. 2008;24:467-472.
- Morris LG, Sikora AG, Hayes RB, Patel SG, Ganly I. Anatomic sites at elevated risk of second primary cancerafter an index head and neck cancer. Cancer, Causes, Control. 2011;22:671-679.
- Anderson DE. The role of genetics in human cancer. CA Cancer J Clin. 1974;24:130-136.
- Frank SA. Genetic predisposition to cancer - insights from population genetics. Nat Rev Genet. 2004;5:764-772.
- Narod SA. Screening for cancer in high risk families. Clin Biochem. 1995;28:367-372.
- Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2(2):e000172.
- Powell S, Tarchand G, Rector T, Klein M. Synchronous and metachronous malignancies: analysis of the Minneapolis Veterans Affairs (VA) tumor registry. Cancer Causes & Control. 2013;24(8):1565-1573.
- Marrano D, Viti G, Grigioni W, Marra A. Synchronous and metachronous cancer of the stomach. Eur J Surg Oncol. 1987;13:493-498.
- Kim JH, Rha SY, Kim C, Kim GM, Yoon SH, Kim KH, et al. Clinic pathologic features of metachronous or synchronous gastric cancer patients with three or more primary sites. Cancer Res Treat. 2010;42:217-224.
- Vogel VG. Identifying and screening patients at risk of second cancers. Cancer Epidemiology and Prevention Biomarkers. 2006;15(11):2027-2032.
|