|
Mariana Silva Costa1, Sara Pinto Lourenço1, Marta Filomena Guimarães1,2,3, Mário Caetano Nora1,2 1Department of General Surgery, Centro Hospitalar de Entre o Douro e Vouga, Santa Maria da Feira, Portugal; 2Endocrine, Cardiovascular & Metabolic Research, Unit for Multidisciplinary Research in Biomedicine (UMIB), University of Porto, Porto, Portugal; 3Department of Anatomy, Institute of Biomedical Sciences Abel Salazar (ICBAS), University of Porto, Porto, Portugal.
Corresponding Author:
Dr. Mariana da Silva Costa Email: mariana87costa@gmail.com
Abstract
Background: The desmoplastic small round cell tumor is a rare disease with poor prognosis. It affects predominantly young males with preferential involvement of the abdominal cavity. Case Report: A 33 year old male presented with right hypochondrium pain, associated with anorexia and weight loss for last 2 months. The imaging studies identified an abdominal mass, without hollow organ association, and multiple liver metastases. The anatomopathological study of the tissue obtained with the percutaneous abdominal mass biopsy confirmed the presence of desmoplastic small round and blue tumor cells. The immunohistochemical and cytogenetic study confirmed the diagnosis. It was proposed for systemic chemotherapy as primary treatment. Conclusion: We intend to emphasize that although this tumor is rare, it should be included in the differential diagnosis of intra-abdominal solid lesions. It also includes a review of the epidemiology, clinical manifestations, as well molecular and pathologic features.
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ff64b02b0000007806000001000800 6go6ckt5b5idvals|969 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
The desmoplastic small round cell tumor is a rare and aggressive entity with specific clinical and pathological features. It was described for the first time by Gerald and Rosai in 1989 [ 1]. It appears mainly in the abdominal cavity of young males, although it was already described in para-testicular, thoracic and intracranial regions [ 2]. It tends to spread over the peritoneum. In the abdomen and pelvis, these tumors appear mainly in advanced stages, standing out as bulky masses, with distant metastasis and peritoneal dissemination [ 3]. The diagnosis is based on histology, immunohistochemistry and cytogenetics. It is associated to specific chromosomal translocations t(11;22) (p13; q12), t(5;19), t(X; 16) and t(4; 10)[ 2, 4, 5, 6]. Histologically it is characterized by the presence of small round blue and undifferentiated cells surrounded by fibrous and desmoplastic stroma. Immunohistochemical findings of these tumor cells expresses cytokeratin and EMA (> 90% of cases), desmin (90%) and neural markers in a variable percentage [ 5, 6]. Most of the clinical and pathological understanding is based on clinical cases and small series of patients. Hence, there are no clear consensus regarding the therapeutic modalities to treat these highly aggressive tumors.The authors’ goal is presenting a clinical case of a young man with a desmoplastic small round cell tumor.
Case Report
A 33-years-old Caucasian male with no significant past history presented continuous and progressive abdominal pain for last 2 months. The pain was ill-defined and confined to the right hypochondrium and was associated with distention of the abdomen. It was associated with anorexia and weight loss. In the physical examination, there was distended abdomen and dull on percussion. For symptomatic relief of abdominal pain, he was treated with a wide range of analgesics, including strong opioids. The full endoscopic study didn’t identify endoluminal lesions, but an abdominal ultrasound identified multiple liver metastases. Then, the contrast-enhanced computed tomography (CT) scan of the thorax, abdomen and pelvis identified hepatosplenomegaly, several abdominal masses without hollow organ association, and multiple liver and bone metastases [Fig.1].
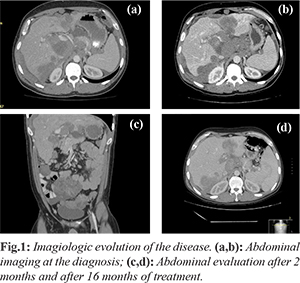
Percutaneous biopsy of the abdominal mass was performed with histopathological evaluation. The microscopical evaluation showed malignant tumor cells arranged in sheets and islands, separated by abundant desmoplastic stroma. The immunohistochemistry was positive for desmin, epithelial membrane antigen, cytokeratin and specific neuronal enolase. Furthermore, the cytogenetic test confirmed the translocationt (11;22) (p13;q12). The above features were compatible with an abdominal desmoplastic small round cell tumor. In view of this diagnosis and inoperable nature of the disease, he was advised combination chemotherapy. He was started on P6 protocol (vincristine, doxorrubicin, cyclophosphamide, ifosfamide and etoposide). After the fourth cycle, ultrasound showed hepatomegaly (20 cm) and ectasia of the intra-hepatic bile duct by extrinsic compression by secondary injuries, splenomegaly (17.7 cm), with moderate peritoneal effusion and peritoneal implants in the right iliac fossa and pelvic cavity. Given the progression of the disease second line chemotherapy was started with topotecan/cyclophosphamide, completing five cycles. It maintains progression of the disease, with progressive worsening of liver function, requiring placement of biliary endoprosthesis (although with poor improvement of bilirubin values). Due to the progression of the disease, he was initiated on palliative chemotherapy of the third line with docetaxel, having performed just one cycle due to frank aggravation of hepatic function. Inevitably, the patient died after 22 months of the diagnosis.
Discussion
The desmoplastic small round cell tumor is a rare and aggressive tumor that belongs to the family of tumors known as small round and blue cells tumors. These include tumors such as Hodgkin's lymphoma, neuroblastoma, rhabdomyosarcoma, Ewing's sarcoma, Wilms tumor and peripheral primitive neuroectodermal tumor [ 2]. The origin of these cells is still unknown, but their predilection for involvement of serosa indicates the possibility of mesothelial origin [ 7, 8]. Several other cases were subsequently described in the international literature. It affects mainly adolescents and young adults, with a 4:1 ratio of men to women [ 6, 7]. In most cases, patients with desmoplastic tumors present with advanced disease. Most remain asymptomatic for extended periods of time, and the diagnosis is made when tumor burden is significant [ 5]. The imaging modality of choice is CT scan with oral and intravenous contrast, usually identifying of one or multiple intra-abdominal masses, with well-defined contours, areas of hypodensity related to foci of necrosis and hemorrhage, intra-peritoneal location and no apparent relationship with hollow organs. The size of those masses varies between 2 and 15 cm in diameter. Ultrasound and MRI do not seem to add any value in the characterization of lesions, although ultrasound may be useful for guiding percutaneous biopsy of more superficial lesions [ 5, 6, 9]. The PET_FDG is a useful test for the identification of occult lesions not identified in the CT and MRI, evaluation of the response to chemo and radiotherapy and early detection of recurrence [ 4, 5, 8, 10]. Core biopsy specimens are preferred to acquire enough samples. Fine-needle aspiration specimens, although commonly employed, are not adequate during the workup of desmoplastic tumors due to issues with low cellularity of the sample, necrosis, and predominantly a desmoplastic reaction. Fine needle aspiration is challenging and requires pathological expertise in the utilization of ancillary techniques such as immunocytochemistry and cytometric immunophenotyping [ 5]. There is a wide range of differential diagnoses, including neoplastic and non-neoplastic diseases, such as peritoneal carcinomatosis, desmoid tumor, fibrous histiocytoma and peritoneal tuberculosis [ 2, 8, 11, 12]. In addition to the macroscopic appearance, the most important diagnostic factor is histopathologic and immunohistochemical expression of a combination of markers for tissue of epithelial, mesenchymal and neuroectodermal origin. The primary mode of dissemination is peritoneal and/or abdominal and pelvic lymph node metastasis. With the progression of the disease, extended tumor implants can be identified throughout the peritoneum, especially on the omentum and mesentery [ 10]. The nodal involvement is described in 50% of patients at presentation. The most common site is the retroperitoneal lymph nodes (80%), followed by the mesenteric and mediastinal region with 20% and 13%, respectively. These lesions are calcified in 20% of cases [ 3]. Distant metastasis is frequent at presentation, ranging from 30 to 50% in different studies. So, the initial staging of such patients should include the screening of metastasis, particularly in the liver, lung and bone [ 3, 4]. In the described case, the presenting symptom was triggered by liver metastasis (pain in the right upper quadrant). In histopathological terms, it is presented with a pattern of small, round, oval or tapered form, with hyperchromatic nuclei, scant cytoplasm and multiple mitosis. They are arranged in nests, cords, parallel, in trabecular or well-defined solid areas involved by desmoplastic stroma cells [ 2, 9]. Immunophenotypically, it is characterized by the expression of epithelial, mesenchymal and neuronal markers. It is positive for desmin (100%), AE1, AE3 (100%), epithelial membrane antigen (100%), cytokeratin (100%) and specific neuronal enolase (100%). The S100 and CD99 markers are partially positive [ 2, 4, 9]. The molecular identification (FISH RT-PCR) confirms, in almost all cases, a specific translocation t(11, 22) (p13; q12) that is juxtaposed to the gene EWSR1 and to the tumor suppressor gene WT1 [ 4, 6]. In this case, all analyzed cells present rearrangement of the WT1 gene (11p13) and of the gene EWSR1 (22p12), according with the diagnosis of desmoplastic small round cell tumor. The semiology of the desmoplastic tumor is nonspecific. It presents with abdominal pain, distended abdomen, dyspepsia and/or vomiting, weight loss, hepatomegaly and ascites, depending on the stage of the disease. It may also be associated with compressive symptoms such as constipation, dysuria, umbilical hernia, intestinal obstruction and others [ 2, 11]. Sometimes the desmoplastic tumor can arise in other primary sites, such as brain, chest, lungs, para-testicular region, ovaries and nasal cavity without specific clinical signs. The treatment options are limited without established treatment protocols. Surgical interventions rarely allow R0 resections. The surgical goal is to remove >90% of the tumor, and resection to less than 1.0 cm tumor size (is mostly requires omentectomy, peritoneal stripping, splenectomy for hilar involvement, and local resection of the diaphragmatic peritoneum) [ 5]. This surgical cytoreduction is possible in 60% of the cases, associated with a better long-term prognosis [ 2, 4]. The cytoreduction followed by systemic chemotherapy with or without radiation therapy has shown the best results in terms of survival [ 3, 8]. The desmoplastic small cell tumors are sensitive to chemotherapy with response rates of approximately 40%, almost temporary [ 9, 13, 14]. Chemotherapy alone is associated with high toxicity and there is weak evidence of long-term survival benefit. Owing to the rarity of this condition chemotherapeutic regimen protocol didn´t exist, although there are several recommended schemes, involving high doses of systemic multidrug therapy that include alkylating agents and the P6 protocol (seven cyclophosphamide courses, doxorubicin, vincristine followed by ifosfamide and etoposide). This last used mainly in resectable tumors [ 13, 14]. In this case, the patient received P6 protocol. The use of a hyper-thermic intra-peritoneal chemotherapy (HIPEC) is being studied and appears to be effective in reducing recurrence [ 7, 11, 14, 15]. Other therapeutic options isolated or in combination with surgery and chemotherapy are being developed but not yet have an established role. These options include abdominal radiotherapy and molecular agents such as imatinib, sunitinib, sorafenib and temsirolimus [ 13].
Conclusion
Most patients have nonspecific symptoms, which delay the diagnosis and worsen the prognosis. Besides, there are few effective treatment protocols. More studies to improve the prognosis of these patients are necessary. It is unlikely that combinational chemotherapy will significantly improve outcomes in desmoplastic tumors. Surgery should remain the cornerstone of treatment. Extended genome sequencing and immunotherapy are being assessed in future clinical trials [ 5]. The development of new therapeutic targets will have a crucial role in altering the course of the disease. Concluding, the desmoplastic small round cell tumor is a rare tumor but it shouldn´t be forgotten as a rare differential diagnosis of intra-abdominal pain.
Contributors: MSC was responsible for the writing the manuscript and the conception of the work; SPL, MFG MCN were responsible for revising it critically. MSC will act as a study guarantor. All authors approved the final version of this manuscript and are responsible for all aspects of this study. Funding: None; Competing interests: None stated.
References - Gerald WL, Rosai J. Case 2: Desmoplasic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9:177-183.
- Briseno-Hernandez A, Quezada-Lopez D, Corona-Cobián LE, Castañeda-Chávez A, Duarte-Ojeda AT, Macías-Amezcua MD. Tumor intra-abdominal desmoplásico de célulaspequenas y redondas. Cirurgia y Cirujanos. 2015;83(3):243-248.
- Thomas R, Rajeswaren G, Eleanor Moskovic E. Desmoplastic small round cell tumour: the radiological, pathological and clinical features. Insights Imaging. 2013;4:111-118.
- Biswa B, Naik V, Shamim E. Intra-abdominal desmoplastic small round cell tumor: Presentation of four cases and review of the literature. Indian J Med Paediatr Oncol. 2010;31:24-27.
- Bulbul A, Fahy B, Xiu J, Rashad S, Asrar Mustafa A, Hatim Husain, et al. Desmoplastic small round blue cell tumor: A review of treatment and potential therapeutic genomic alterations. Sarcoma 2017; ID 1278268.
- Jordan A, LaQuaglia M, Modak S. Management of desmoplastic small round cell tumor. Semin Pediatr Surg. 2016;25:299-304.
- Hirano G, Irie M, Nakashima Y, Shakado S, Sohda T, Tanaka T, et al. Desmoplastic small round cell tumors in a young man. Intern Med. 2013;52:1909-1914.
- Ashrafi M, Saffar H, Mirsharifi SR, Seyed MT. Intra-abdominal desmoplastic small round celltumor in a 45-year-old man: A case report. Acta Medica Iranica. 2013;51(8):583-586.
- Mora J, Modak S, Cheung NK, Meyers P, de Alava E, Kushner B, et al. Desmoplastic small round cell tumor 20 years after its discovery. Future Oncol. 2015;11(7):1071-1081.
- Kis B, O'Regan KN, Agoston A, Javery O, Jagannathan J, Ramaiya NH. Imaging of desmoplastic small round cell tumors in adults. The British Journal of Radiology. 2012;85:187-192.
- Xiang L, Jing Y, Zhao J. Desmoplastic small round cell tumor: a case report and review of the literature. World Journal of Surgical Oncology. 2014;12:9.
- Chen J, Sheng J, Wang L, Wang ZM, Li L. Desmoplastic small-round-cell tumor of the abdomen: A report of two rare cases. Oncology Letters. 2015;10:705-708.
- Wong HH, Hatcher HM, Benson C, Al-Muderis O, Horan G, Fisher C, et al. Desmoplastic small round cell tumor: characteristic and prognostic factors of 41 patients and review of the literature. Clinical Sarcoma Research. 2013;3:14.
- Benhammane H, Chbani L, Ousadden A, Mouquit O. Desmoplastic small round cell tumor of the abdomen: A case report and literature review of therapeutic options. Health. 2012;4:207-211.
- Honoré C, Atallah V, Mir O, Orbach D, Ferron G, LePéchoux C, et al. Abdominal desmoplastic small round cell tumor without extraperitoneal metastases: Is there a benefit for HIPEC after macroscopically complete cytoreductive surgery? PLoS ONE. 2017;12:e0171639.
|