6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffa4cf2b0000009c08000001000100
6go6ckt5b5idvals|970
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Incidence of pheochromocytoma or paraganglioma during pregnancy is estimated to occur in 0.007% of all pregnancies [
1]. Due to its rarity, management is often inferred from case studies and expert opinion. We describe the peri-operative challenges and anaesthetic management of a 36-year-old woman diagnosed with functioning paraganglioma and pre-eclampsia on a background of chronic hypertension, presenting with a twin pregnancy after in-vitro fertilization. This patient presents both a diagnostic and management challenge, and highlights the importance of considering overlapping pathologies in a patient with resistant hypertension. This is the first described early presentation of concomitant paraganglioma and preeclampsia in a parturient and highlights how its diagnosis and subsequent multidisciplinary management with obstetricians, endocrinologists and intensivists resulted in favourable maternal and fetal outcome. We also describe our anaesthetic technique and anticipated anesthetic complications as well as how we addressed them.
Case Report
A 36-year-old Chinese primigravida, presented at 10 weeks gestational age on routine antenatal visit, after successful in-vitro fertilization. She had a 10-year history of hypertension on oral nifedipine LA 60 mg bd. Ultrasound Doppler revealed a viable twin pregnancy. At gestational week 12, she presented with hypertensive crisis of 180/94 mmHg, episodic headaches and sinus tachycardia of 120 beats per minute. Thyroid function tests were normal. Ultrasound kidney was unremarkable. A 2-D echocardiogram revealed a left ventricular ejection fraction of 70-75% with no valvulopathies.
A differential diagnosis of phaeochromocytoma was considered. 24-hour urine norepinephrine level was 796 nmol [Normal (N): 89-473 nmol/day], dopamine 8450 (N: 424-2612 nmol/day), metanephrine 1323 (N: 400-1500 nmol/day), normetanephrine 5378 (N: 6000-1900 nmol/day). A non-contrast abdominal MRI at gestational week 14 was unremarkable. The likely diagnosis of paraganglioma was suspected and she was followed up closely in the high risk maternal-fetal clinic by the obstetrician and endocrinologist. She was also started on phenoxybenzamine 20 mg twice daily. At gestational week 33, she was readmitted for uncontrolled hypertension. Investigations revealed elevated uric acid levels up to 777 umol/L, urine total protein 6.1 g/L (N: 0.01-0.14 g/L) and a mild transaminitis suggestive of concomitant preeclampsia. PO labetalol 200 mg tds was started but she remained hypertensive with episodic headaches. Intravenous magnesium sulphate infusion was started at 1 g/hour. Decision was made for urgent caesarean section in view of impending eclampsia. Her height and weight was 150 cm and 75 kg respectively. Two 18G intrave-nous cannulas, and a 20G radial intra-arterial line was inserted pre-induction. Emergency medications were prepared including IV phentolamine and IV esmolol. Pre-induction blood pressure was 170/100 mmHg with a heart rate of 90 beats/min. She was given divided doses of esmolol 10 mg to a total of 90 mg to attain BP of 150/80 mmHg. IV magnesium sulphate 1g/hour infusion was continued. A rapid sequence induction and cricoid pressure with thiopentone 375 mg, succinylcholine 100 mg and IV atracurium 30 mg, target controlled infusion remifentanil was started at 4 ng/ml, increased to 6 ng/ml during intubation, and maintained at 4 ng/ml during the surgery. Airway was secured using C-MAC videolaryngoscope #3 Mackintosh blade with a #7.0 oral endotracheal tube. Anaesthesia was maintained on nitrous oxide:oxygen 50:50, and end-tidal sevoflurane concentration of 1.5%.
Twins weighing 2.283 kg and 1.786 kg were delivered. Twin one was sent to neonatal intensive care unit for ventilatory support. Twin two was sent to special care nursery for monitoring due to low birth weight. Oxytocin (3 units) was then given as a slow bolus and continued as an oxytocin infusion of 30 units over 4 hours. Post-delivery, her systolic blood pressure dropped to 50-60 mmHg. IV magnesium sulphate was temporarily discontinued. A total of 40 mg of ephedrine and 1000 mcg of phenylephrine was administered promptly to achieve MAP >55 mmHg. Her BP subsequently responded to 1L of gelafundin, maintaining systolic 100 mmHg. Neuromuscular blockade was reversed with neostigmine 2.5 mg and atropine 0.9 mg. She was extubated awake and transferred to the intensive care unit for further monitoring. Post-operatively, she was continued on PO nifedipine LA 60 mg twice daily, PO atenolol 25 mg, phenoxybenzamine 20 mg twice daily and IV magnesium sulfate infusion for 24 hours. On 3rd post-operative day, she was discharged from the intensive care unit.
Discussion
Pheochromocytomas and paraganglioma (PGL) are neuroendocrine tumors derived respectively, from adrenal chromaffin cells and extra-adrenal paranglia, and estimated to occur in 0.007% of all pregnancies. Overall maternal and fetal mortality is said to be 3.6% and 12% respectively [
1]. Hypertension, gestational or chronic, occurs in 3.6% to 9.1% of pregnancies [
2]. Particularly, this patient highlights the possibility of dual pathologies presenting in a parturient. Although
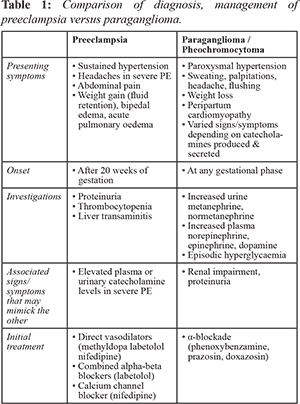
Table 1 illustrates the differences between preeclampsia (PE) and PGL in presentation, often these symptoms are variable and non-specific hence demanding a high clinical suspicion and low threshold to investigate. Undiagnosed catecholamine secreting tumors (CST) may have a detrimental outcome as management differs. While labetolol is a recommended first-line therapy for hypertension in parturients, oral labetolol has a 3:1 beta:alpha blockade and starting beta-blockade in a patient with CST could result in unopposed alpha-adrenergic receptor stimulation that could precipitate a hypertensive crisis.
The management of BP in a parturient with CST requires a fine balance between adequate catecholamine blockade and maintaining utero-placental perfusion. Alpha-blockade is typically first-line as norepinephrine is predominantly secreted and guided by Roizen criteria [
3] to determine adequacy of alpha-blockade in non-pregnant patients. In a parturient, there is no known target blood pressure and hypotension may result in insufficient utero-placental perfusion. Conversely, hypertensive surges may compromise the uteroplacental circulation, leading to intrauterine growth regardation and fetal demise [
4]. Compounded by preeclampsia which suggests abnormal placentation, impaired spiral arteries invasion and placental hypoperfusion, it is prudent to accept a higher target of < 150/100 mmHg or < 140/90 mmHg if end-organ involvement is present [
5]. Starting alpha-blockers has its own risks as drugs such as phenoxybenzamine can transfer across the placenta and accumulate in fetal blood. Newborns are at risk of respiratory depression and hypotension, and must be observed closely after delivery [
6]. The definitive treatment for preeclampsia is the delivery of the fetus.
Furthermore, there are vast differences in patient management between PE and CST, and hence dual pathologies demand weighing the pros and cons of both. CSTs discovered in the first 24 weeks of gestation should be removed preferably by a laparascopic approach in the second trimester; whereas CSTs discovered in the third trimester should delay surgical excision till fetus is viable and able to be delivered, with the tumor being removed either immediately after delivery or at a later date [
7]. Conversely, anti-hypertensive therapy and magnesium sulphate should be started for patients with severe PE as a temporizing measure, and the definitive treatment is delivery. In our patient where the PGL was not located, compounded by PE, we recommend that management should be individualized based on the i) nature of the catecholamine-secreting tumour (CST), ii) gestational age iii) patient’s wishes; and undertaken jointly by a multidisciplinary team of obstetricians, endocrinologists and anaesthesiologists.
The use of spinal anaesthesia in LSCS to obtund sympathetic stimulation from pain during LSCS has been described in literature. However, our decision to proceed under general anaesthesia was in view of i) airway control in view of the potentially difficult airway in preeclampsia secondary to airway edema and ii) expected hemodynamic instability. Normalizing blood pressure, heart rate and restoring intravascular volume are the main targets in the pre-operative management of PGL [
7] as patients on alpha-blockers are typically volume-deplete but with concomitant pre-eclampsia, judicious fluid administration is advisable to avoid precipitating acute pulmonary edema. Our anaesthetic goals are mainly to maintain hemodynamic stability. Intra-operative catecholamine surges should be anticipated and suppressed during laryngoscopy and tracheal intubation, surgical stimulation and fundal pressure. This should be performed by ensuring adequate depth of anaesthesia and use of drugs such as remifentanil infusion for quick titration to these stimulating events. Remifentanil is used in spite of its ability to cross the placenta as it is rapidly metabolized and redistributed in the fetus. Invasive intra-arterial cannulation is necessary for tight control of blood pressure. Large bore IV cannulation should be established before induction for fluid resuscitation if necessary.
Furthermore, drugs that may precipitate sympathetic crisis should be avoided. Particular to PGLs, avoidance of glucocorticoids may be essential as its administration may precipitate a catecholamine crisis as paragangliomas are postulated to be unlikely to secrete epinephrine, as the enzyme phenylethanolamine N-methyltransferase (PNMT), which converts norepinephrine to epinephrine, requires cortisol as a cofactor.
Conclusion
The primary goals of CST and PE in pregnancy are early diagnosis and avoidance of sympathetic surge. A multidisciplinary approach should be conducted and possibility of multiple pathologies should be considered especially in resistant hypertension.
Contributors: Lim ML and Yek JLJ conceived the presented idea, acquired and interpreted the data required as well as drafted and revised the manuscript in consultation with Selvan S. All authors have read and approved the final manuscript as well as provided critical feedback, discussed and are accountable for all aspects of the final manuscript in ensuring its accuracy and integrity are appropriately investigated and resolved.
Funding: None; Competing interests: None stated.
References
- Wing LA, Conaglen JV, Meyer-Rochow GY, Elston MS. Paraganglioma in pregnancy: A case series and review of the literature. The Journal of Clinical Endocrinology & Metabolism. 2015;100(8):3202-3209.
- Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open. 2011;1(1):e000101.
- Roizen MF, Horrigan RW, Koike M, Eger IE, 2nd, Mulroy MF, Frazer B, et al. A prospective randomized trial of four anesthetic techniques for resection of pheochromocytoma. Anesthesiology. 1982;57:A43.
- van der Weerd K, van Noord C, Loeve M, Knapen MFCM, Visser W, de Herder WW, et al. Pheochromocytoma in pregnancy: case series and review of literature. Eur J Endocrinol. 2017;177 (2):R49-R58.
- NICE Guideline 107: hypertension in pregnancy. Available at: www.nice.org.uk/ guidance/CG107. August 2010. Accessed on August 18, 2019.
- Santeiro ML, Stromquist C, Wyble L. Phenoxybenzamine placental transfer during the third trimester. Ann Pharmacother. 1996;30(11):1249-1251.
- Lenders JW. Pheochromocytoma and pregnancy: a deceptive connection. Eur J Endocrinol. 2012;166:143-150.