6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffb0462d0000004409000001000800
6go6ckt5b5idvals|990
6go6ckt5b5|2000F757Tab_Articles|Fulltext
Introduction
Uterine artery embolization (UAE) was first described in 1995 for the treatment of uterine fibroids [
1]. Besides its popularity among the treatment of symptomatic fibroids, the UAE has been developing in the safer and minimally invasive treatment of adenomyosis for alleviating the disabling symptoms with or without fibroids and preserving uterus without scars in female patients. However, concerns about fertility [
2] or the complications during the next pregnancy post UAE remain to be answered. Here we present a rare case of spontaneous uterine rupture at 24 weeks of gestation after UAE due to adenomyosis.
Case Report
A 35 years old woman (gravid 2, para 0) underwent UAE in 2013 because of endometrioma accompanied with progressive dysmenorrhea for 3 years. She was clinically diagnosed with adenomyosis in the posterior wall of uterus, with pre-surgery contrast-enhanced magnetic resonance imaging (MRI) showing that the uterus was spherical and significantly enlarged, about 6.3×6.0×5.9 cm in size and the muscular layer of the posterior wall of the uterus was thickened locally, up to 4.3 cm at the thickest point. The patient was offered UAE through bilateral uterus artery as a conservative therapy. The follow-up after 3 months showed obvious relief of the menstrual pain and the shrunken size of uterus to 5.4×3.8×5.7 cm without any surgery related complaints after UAE [Fig.1].
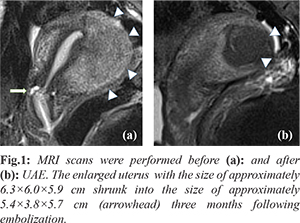
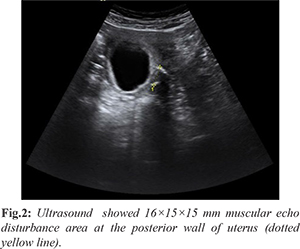
During the next 5 years until her pregnancy (gravid 3, para 0), the patient had regular menstrual period, without histories of infertility, invasive surgery or interventional therapy due to adenomyosis or other gynecological disease. She went regular antenatal care in our hospital and during 8 to 10 weeks of gestation, the patient complained about occasional soreness of waist and abdominal pain with few vaginal bleedings. The ultrasound detected a 16×15×15 mm muscular echo disturbance area at the posterior wall of uterus at 11th gestational week, which was suspected to be a nodule of endometrioma [Fig.2]. Hemoglobin was 122 g/L from the 24th week’s blood test and until then no symptoms or signs indicating anemia.
At 24th gestational week, sudden continuous abdominal pain occurred and the patient was sent to an emergency surgery in local hospital. Abdominal tenderness existed and bedside ultrasound indicated the bradycardia of the fetus and medium volume of abdominal and pelvic fluid accumulated so that there was an internal bleeding. Primary clinical diagnosis was hemorrhagic shock and fetal distress. Laparatomy showed 7 cm laceration at the posterior wall of the uterus, surrounded by endometrioma-like necrotic tissue with partial placenta implantation in posterior wall of the uterus. Fetal amniotic sac and part of the placenta were found in the abdominal cavity during the operation, without fetal vital sign. Operative incision was in continuous bleeding and accumulated hemorrhage was up to 5000 ml, so finally, hysterectomy plus bilateral salpingectomy was performed.
The patient recovered well and was discharged from the hospital on the 9th day. The pathological diagnosis of the removed tissue was placenta accreta, leiomyomatoid tissue with collagenization and calcification separated from the laceration and local necrotic tissue.
Discussion
Uterine rupture during pregnancy after UAE are rare and severe and this is the third according to the previous published work (MEDLINE; 1966 to July 2019; search terms: ‘rupture’ ’UAE’ and ‘pregnancy’) [
3-
5]. Furthermore, the ruptured sites of these cases are almost the same which is at the back or fundus of uterus. We barely make sure when the rupture occurred specifically because it could happen during each term of pregnancy and even without pregnancy.
Generally uterine rupture is a deadly complication which would occur to those who had uterine scars from cesarean delivery, uterine instrumentation or trauma. Besides its wide application in the treatment of symptomatic fibroids, UAE has become prevalent in postpartum hemorrhage (PPH) and adenomyosis due to its safety and minimal invasion. However, correlations between UAE and reproductive health are rarely documented, let alone uterine rupture. Here we provide another evidence which describes unscarred uterine rupture during pregnancy in a patient after UAE, besides one due to cervical ectopic pregnancy [
3] and the other for placenta polyps [
4] with twice UAE. Additionally, one previous case report described a uterine rupture 3 months after UAE for multiple symptomatic myoma in a non-pregnant woman [
5]. However, we are the first one to report a case of uterine rupture after UAE associated with adenomyosis at the posterior wall of uterus. Moreover, it has been 6 years after the UAE when the uterine rupture occurred.
All of the three cases of uterine rupture were postpartum diagnosis without predictable symptoms or specific signs until the acute abdominal pain which led to an emergency laparotomy. Plus, the tear at the back wall of the womb hardly has any signs and lacks specific imaging examination. One case showed ischemic area after UAE through MRI during the 3-months follow-up, still imaging showed only myometrial thinning at the fundus of the uterus during the 35th week of gestation. Even so, we still missed some key details indicated uterine rupture like mild abdominal pain during the early gestation, unconfirmed lesion which was similar to myoma degeneration according to the ultrasound, undetected placenta accreta and lack of MRI examination. In terms of above, signs like soreness of waist or abdominal pain during the pregnancy should be alerted in case of uterine rupture. Besides, as for a highly suspected uterus rupture, MRI should be taken to evaluate the uterus blood supply. Most uterine rupture occurred 3 months after surgery, and this case suggests that rupture is still possible 6 years later.
We questioned why is the posterior wall teared in all three cases so that we found: i) UAE is performed to block the bilateral uterine arteries which may contribute more to posterior uterus where few collateral arterial branches exist, ii) local ischemic and hypoxia environment due to UAE exacerbates tissue necrosis to achieve therapeutic effect, iii) the ischemic area [
3] or the original lesion (placenta polyps [
4] or endometrioma and necrotic tissue) all happened on the posterior wall. Plus, myometrium in these cases was extremely thin. One report indicates the most common rupture sites were the cornual area and the uterine fundus [
6]. More anatomic evidences should be provided to explain our hypothesis. Another doubt is whether the rupture was actually due to placenta accreta in the posterior wall of uterus. No existing evidence can verify the causal relationship between accreta and rupture because most accreta occurred to scarred uterus. What’s more, it was conducted that the accreta within unscarred uterus in our case was due to the ischemic area resulting from UAE.
Even though several reviews and case control studies illustrate the favorable outcomes of UAE to adenomyosis, for example, after 7 years of follow-up, 82% of patients results in preservation of the uterus and 74% seem to respond well to UAE [
1] or symptoms relief and a short-term uterine volumes decrease, especially in vascular lesions [
7] etc. However some earlier studies considered it lower efficacy in adenomyosis [
8]. Up to date, randomized controlled trials are still going on and one of them set up as “Quality of Life after Embolization vs Hysterectomy in Adenomyosis” (QUESTA) trial [
9].
Conclusion
A high index of suspicion for uterine rupture is required following UAE in patients presenting with abdominal pain.
Contributors: Li collected the data and Cui wrote the manuscript. Zhou and Li critically revised for important intellectual contents. He Zhang and Guofu Zhang contributed to the radiological imaging. Li will act as a study guarantor. All authors have read and approved the final manuscript and are responsible for all aspects of study.
Funding: None; Competing interests: None stated.
References
- de Bruijn Annefleur M, Smink Marieke, Hehenkamp Wouter JK, et al. Uterine artery embolization for symptomatic adenomyosis: 7-year clinical follow-up using UFS-Qol questionnaire. Cardiovasc Intervent Radiol, 2017:40:1344-1350.
- Lohle Paul NM, Higué David, Herbreteau Denis. Uterine artery embolisation in women with symptomatic adenomyosis. Presse Med. 2019:48:435-439.
- Takeda Jun, Makino Shintaro, Ota Atsuyuki, Tawada T, Mitsuhashi N, Takeda S. Spontaneous uterine rupture at 32 weeks of gestation after previous uterine artery embolization. J Obstet Gynaecol Res. 2014;40:243-246.
- Ando Miho, Goto Maki, Matsuoka Sakiko, To Y, Eguchi F, Tsijioka H. Case of uterine rupture after multiple intrauterine operations and uterine artery embolization. J Obstet Gynaecol Res. 2019;45:734-738.
- Shashoua Abraham R, Stringer Nelson H, Pearlman Julie B, Behmaram M, Stringer EA. Ischemic uterine rupture and hysterectomy 3 months after uterine artery embolization. J Am Assoc Gynecol Laparosc. 2002;9:217-220.
- Posthumus L, Donker ME. Uterine rupture in a primigravid patient, an uncommon but severe obstetrical event: a case report. J Med Case Rep. 2017;11:339.
- Dessouky R, Gamil Sherif A, Nada MG, Mousa R, Libda Y. Management of uterine adenomyosis: current trends and uterine artery embolization as a potential alternative to hysterectomy. Insights Imaging. 2019;10:48.
- Rabinovici J, Stewart EA. New interventional techniques for adenomyosis. Best Pr Res Clin Obs Gynaecol. 2006;20:617-636.
- de Bruijn Annefleur Machteld, Lohle Paul Nm, Huirne Judith Af, Twisk M, De Varies J, Hehenkamp WJ, et al. Uterine artery embolization versus hysterectomy in the treatment of symptomatic adenomyosis: Protocol for the Randomized QUESTA Trial. JMIR Res Protoc. 2018;7.